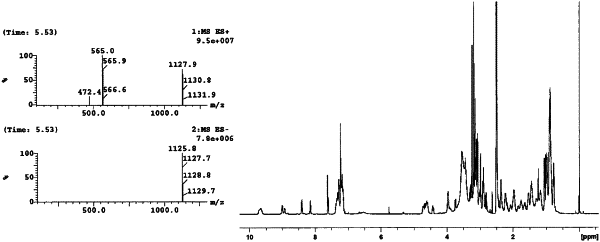
| CPC A61K 47/6849 (2017.08) [A61K 38/07 (2013.01); A61K 38/12 (2013.01); A61K 47/545 (2017.08); A61K 47/6809 (2017.08); A61K 47/6811 (2017.08); A61P 35/00 (2018.01)] | 18 Claims |
|
1. A linker-drug conjugate of the following formula (II):
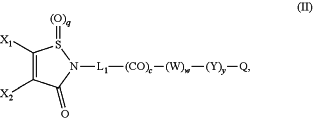 or a salt thereof,
wherein:
X1 and X2 represent, independently of each other, H, a halogen atom, a (C1-C6)alkoxy, an optionally substituted aryloxy, or —O—(CH2CH2O)rH, provided that X1 and X2 do not represent H at the same time;
L1 represents a group of formula L1′-(CO—Z′)z′ with L1′ being —(CH2)n—, —(CH2CH2O)m—CH2—CH2—, arylene, heteroarylene, cycloalkanediyl, —(CH2)n-arylene-, —(CH2)n-heteroarylene-, —(CH2)n-cycloalkanediyl-, -arylene-(CH2)p—, -heteroarylene-(CH2)p—, -cycloalkanediyl-(CH2)p—, —(CH2)n-arylene-(CH2)p—, —(CH2)n-heteroarylene-(CH2)p—, —(CH2)n-cycloalkanediyl-(CH2)p—, —(CH2CH2O)m—CH2—CH2-arylene-(CH2)p—, —(CH2CH2O)m—CH2—CH2-heteroarylene-(CH2)p—, —(CH2CH2O)m—CH2—CH2-cycloalkanediyl-(CH2)p—, —(CH2)n-arylene-CH2—CH2—(OCH2CH2)m—, —(CH2)n-heteroarylene-CH2—CH2—(OCH2CH2)m—, or —(CH2)n-cycloalkanediyl-CH2—CH2—(OCH2CH2)m—;
each W independently represents an amino acid unit;
Y is PAB-CO—(Z)z—, with PAB being
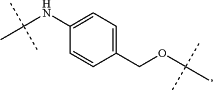 the oxygen of the PAB unit being linked to CO—(Z)z;
Z is —NR4—(CH2)u—NR5—, —NR4—(CH2)u—NR5—CO—, —NR4—(CH2)u—NR5—CO—(CH2)v—, or —NR4—(CH2)u—NR5—CO—(CH2)v—CO—, the NR4 group being linked to the CO group of PAB-CO;
Z′ is —NR4—(CH2)u—NR5— or —NR4—(CH2)u—NR5—CO—(CH2)v—, the NR4 group being linked to the CO group of CO—Z′;
R4 and R5 are independently H or a (C1-C6)alkyl group;
Q is a residue of an auristatin, an anthracycline, camptothecin, SN-38, a tubulysin, a calicheamicin, a maytansinoid, a duocarmycin, an amanitine, a pyrrolobenzodiazepine, or an activator of immune check point;
c is 0 or 1;
m is an integer from 1 to 15;
n is an integer from 1 to 6;
p is an integer from 1 to 6;
q is 0, 1 or 2;
r is an integer from 1 to 24;
u is an integer from 1 to 6;
v is an integer from 1 to 6;
w is an integer from 0 to 5;
y is 0 or 1;
z is 0 or 1; and
z′ is 0 or 1.
|