| CPC A61K 31/506 (2013.01) [A61K 45/06 (2013.01); A61P 35/00 (2018.01); C07D 403/12 (2013.01); C07D 403/14 (2013.01); C07D 413/14 (2013.01); C07D 417/14 (2013.01)] | 23 Claims |
|
1. A method of treatment by antagonizing prostaglandin 2 receptors EP2 and/or EP4 of pain; endometriosis; autosomal dominant polycystic kidney disease; or pneumonia; or for the control of female fertility; comprising administering to a subject in need thereof a compound of formula (I), or a pharmaceutically acceptable salt thereof,
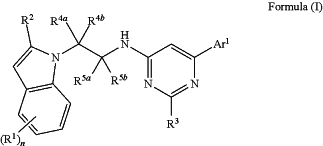 (R1)n represents one, two or three optional substituents on the indole ring, wherein said substituents are independently selected from (C1-3)alkyl, (C1-3)alkoxy, halogen, (C1-3)fluoroalkyl, (C1-3)fluoroalkoxy, or cyano; or two R1 together form a group —O—CH2—O—, and the remaining R1, if present, represent halogen;
R2 represents (C1-4)alkyl, halogen, or cyano;
R3 represents hydrogen, methyl or trifluoromethyl;
R4a and R4b independently represent hydrogen, methyl, or R4a and R4b together with the carbon atom to which they are attached represent a cycloprop-1,1-diyl group;
R5a and R5b independently represent hydrogen, methyl, or R5a and R5b together with the carbon atom to which they are attached represent a cycloprop-1,1-diyl group;
Ar1 represents
phenyl, or 5- or 6-membered heteroaryl; wherein said phenyl or 5- or 6-membered heteroaryl independently is mono-, di- or tri-substituted, wherein the substituents are independently selected from
(C1-6)alkyl;
(C1-4)alkoxy;
(C1-3)fluoroalkyl, wherein said (C1-3)fluoroalkyl is optionally substituted with hydroxy;
(C1-3)fluoroalkoxy;
halogen;
cyano;
(C3-6)cycloalkyl, wherein said (C3-6)cycloalkyl is unsubstituted or mono-substituted with amino;
(C4-6)cycloalkyl containing a ring oxygen atom, wherein said (C4-6)cycloalkyl containing a ring oxygen atom is unsubstituted or mono-substituted with fluoro, hydroxy, or methoxy;
(C3-6)cycloalkyl-oxy;
hydroxy;
nitro;
B(OH)2;
2,2,2-trifluoro-1,1-dihydroxy-ethyl;
X1—CO—RO1, wherein
X1 represents a direct bond, (C1-3)alkylene, —O—(C1-3)alkylene-*, —NH—(C1-3)alkylene-*, —S—CH2—*, —CF2—, —CH═CH—, —C≡C—, —NH—CO—*, —CO—, or (C3-5)cycloalkylene; wherein the asterisks indicate the bond that is linked to the —CO—RO1 group; and
RO1 represents
—OH;
—O—(C1-4)alkyl;
—NH—SO2—RS3 wherein RS3 represents (C1-4)alkyl, (C3-6)cycloalkyl wherein the (C3-6)cycloalkyl optionally contains a ring oxygen atom, (C3-6)cycloalkyl-(C1-3)alkylene wherein the (C3-6)cycloalkyl optionally contains a ring oxygen atom, (C1-3)fluoroalkyl, phenyl, or —NH2;
O-phenyl;
O—CH2—CO—RO4, wherein RO4 represents hydroxy, or (C1-4)alkoxy, or —N[(C1-4)alkyl]2;
O—CH2—O—CO—RO5, wherein RO5 represents (C1-4)alkyl or (C1-4)alkoxy;
O—CH2—CH2—N[(C1-4)alkyl]2; or
(5-methyl-2-oxo-[1,3]dioxol-4-yl)-methyloxy-;
—CO—CH2—CN;
—CO—CH2—OH;
—CO—H;
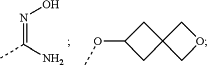 2-hydroxy-3,4-dioxo-cyclobut-1-enyl;
hydroxy-(C1-4)alkyl;
dihydroxy-(C2-4)alkyl;
hydroxy-(C2-4)alkoxy;
(C1-4)alkoxy-(C2-4)alkoxy;
(CH2)m—NRN1RN2, wherein m represents the integer 0 or 1; and wherein
RN1 and RN2 independently represent hydrogen, (C1-4)alkyl, (C1-4)alkoxy-(C2-4)alkyl, (C3-6)cycloalkyl, (C2-3)fluoroalkyl, -or —SO2—(C1-4)alkyl;
or RN1 independently represents hydrogen or (C1-4)alkyl, and RN2 independently represents —CO—H, —CO—(C1-3)alkyl, —CO—(C1-3)alkylene-OH, or —CO—O—(C1-3)alkyl;
or RN1 and RN2 together with the nitrogen to which they are attached form a 4-, 5- or 6-membered saturated ring optionally containing one ring oxygen or ring sulfur atom, wherein said ring is unsubstituted, or mono-substituted with oxo on a ring carbon atom, or disubstituted with oxo on a ring sulfur atom;
—CO—NRN3RN4 wherein RN3 and RN4 independently represent hydrogen, (C1-4)alkyl, hydroxy-(C2-4)alkyl, (C1-3)alkoxy-(C2-4)alkyl, dimethylamino-(C2-4)alkyl, (C1-4)alkoxy, hydroxy-(C2-4)alkoxy, benzyloxy, or hydroxy;
—NH—CO—NRN5RN6 wherein RN5 and RN6 independently represent hydrogen or (C1-4)alkyl;
SO2—RS1 wherein RS1 represents hydroxy, (C1-4)alkyl, or —NRN7RN8 wherein RN7 and RN8 independently represent hydrogen or (C1-3)alkyl;
S—RS2 wherein RS2 represents (C1-4)alkyl, (C3-6)cycloalkyl, or 2-fluoro-vinyl;
5-oxo-4,5-dihydro-[1,2,4]oxadiazol-3-yl, or 3-oxo-2,3-dihydro-[1,2,4]oxadiazol-5-yl;
phenyl-oxy, wherein the phenyl is optionally mono-substituted with halogen;
benzooxazol-2-yl; or
(CH2)p-HET, wherein p represents the integer 0 or 1; and wherein HET represents a 5- or 6-membered heteroaryl, wherein said 5- or 6-membered heteroaryl is unsubstituted, or mono- or di-substituted, wherein the substituents are independently selected from (C1-4)alkyl, (C1-4)alkoxy, —COOH, hydroxy, fluoro, 2-amino-2-oxo-ethyl, 2-carboxy-ethyl, (C3-5)cycloalkyl, or —NRN9RN10 wherein RN9 and RN10 independently represent hydrogen or (C1-3)alkyl;
or Ar1 represents 8- to 10-membered bicyclic heteroaryl; wherein said 8- to 10-membered bicyclic heteroaryl independently is unsubstituted, mono-, di- or tri-substituted, wherein the substituents are independently selected from (C1-4)alkyl; (C1-4)alkoxy; (C1-3)fluoroalkyl; (C1-3)fluoroalkoxy; halogen; cyano; hydroxy, or —(C0-3)alkylene-COORO2 wherein RO2 represents hydrogen or (C1-4)alkyl;
or Ar1 represents 8- to 10-membered partially aromatic fused bicyclic heterocyclyl comprising one to four heteroatoms independently selected from nitrogen, oxygen and sulfur; wherein said 8- to 10-membered heterocyclyl is linked to the rest of the molecule at the aromatic ring moiety; wherein said 8- to 10-membered heterocyclyl independently is unsubstituted, mono-, or di-substituted, wherein the substituents are independently selected from oxo, (C1-6)alkyl, and —(C0-3)alkylene-COORO3 wherein RO3 represents hydrogen or (C1-3)alkyl.
|