| CPC A61K 31/4545 (2013.01) [A61K 31/165 (2013.01); A61K 31/357 (2013.01); A61K 39/39541 (2013.01); A61P 35/00 (2018.01)] | 10 Claims |
|
1. A method of treating colon cancer in a human, said method comprising administering an effective amount of a small molecule C-C Chemokine Receptor 4 (CCR4) inhibitor and an anti-CTLA-4 antibody,
wherein the CCR4 inhibitor has the following formula (If)
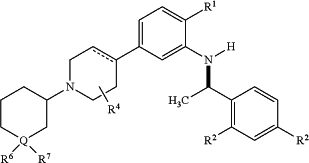 or a pharmaceutically acceptable salt thereof, wherein
R1 is halogen;
each R2 is halogen;
R4 is hydrogen;
Q is N;
R6 is alkylene-C(O)ORa;
wherein
Ra is hydrogen; and
R7 is absent.
|