| CPC A61K 31/167 (2013.01) [A61K 31/337 (2013.01); A61K 31/34 (2013.01); A61K 31/351 (2013.01); A61K 31/357 (2013.01); A61K 31/397 (2013.01); A61K 31/401 (2013.01); A61K 31/4015 (2013.01); A61K 31/415 (2013.01); A61K 31/4164 (2013.01); A61K 31/4192 (2013.01); A61K 31/421 (2013.01); A61K 31/426 (2013.01); A61K 31/4409 (2013.01); A61K 31/4439 (2013.01); A61K 31/47 (2013.01); A61K 31/513 (2013.01); A61K 31/7028 (2013.01); A61P 31/20 (2018.01); C07C 303/00 (2013.01); C07C 317/44 (2013.01); A61K 45/06 (2013.01); C07B 2200/05 (2013.01); C07C 2601/02 (2017.05); C07C 2601/04 (2017.05); C07C 2601/08 (2017.05); C07C 2601/10 (2017.05); C07C 2601/14 (2017.05); C07C 2601/16 (2017.05); C07C 2602/44 (2017.05); C07C 2602/50 (2017.05)] | 17 Claims |
|
1. A pharmaceutical composition comprising a compound represented by the formula
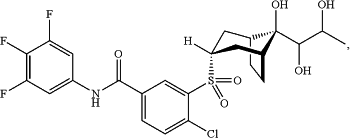 wherein R is hydrogen or fluorine;
and a pharmaceutically acceptable carrier or excipient.
|