| CPC C07D 498/04 (2013.01) [C07D 498/14 (2013.01)] | 113 Claims |
|
1. A compound of Formula I or a pharmaceutically acceptable salt thereof:
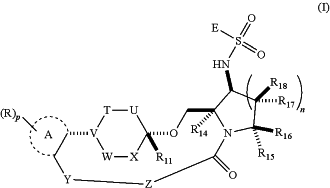 wherein:
ring A is selected from the group consisting of phenyl, pyridinyl, pyridazinyl, pyrimidinyl, pyrazinyl, and triazinyl;
n is 1, 2, or 3;
E is selected from the group consisting of NRaRb, C1-C3 alkylene-NRaRb, C1-C3 alkyl, C2-C4 alkenyl, C2-C4 alkynyl, C3-C8 cycloalkyl, C1-C3 alkylene-(C3-C8 cycloalkyl), 4- to 10-membered heterocyclyl, C1-C3 alkylene-(4- to 10-membered heterocyclyl), C6-C10 aryl, C1-C3 alkylene-(C6-C10 aryl), 5- to 10-membered heteroaryl, and C1-C3 alkylene-(5- to 10-membered heteroaryl), wherein the C1-C3 alkylene-NRaRb, C1-C3alkyl, C2-C4 alkenyl, C2-C4 alkynyl, C3-C8 cycloalkyl, C1-C3 alkylene-(C3-C8 cycloalkyl), 4- to 10-membered heterocyclyl, C1-C3 alkylene-(4- to 10-membered heterocyclyl), C6-C10 aryl, C1-C3 alkylene-(C6-C10 aryl), 5- to 10-membered heteroaryl, or C1-C3 alkylene-(5- to 10-membered heteroaryl) is unsubstituted or substituted with one or more halogen, hydroxyl, C1-C3 alkyl, or C1-C3 alkoxyl;
T is CR1R2 or O;
W is CR4R5 or O;
U is CR6R7;
X is CR8R9;
V is CR3 or N;
Y is O;
Z is (CR12R13)m;
each R is, independently, selected from the group consisting of halogen, deuterium, hydroxyl, cyano, unsubstituted C1-C3 alkyl, and C1-C3 alkyl substituted with one or more halogen or deuterium;
p is 0, 1, 2, 3, or 4;
Ra and Rb are each, independently, H or unsubstituted C1-C3 alkyl;
m is 1;
and further wherein:
R1, R2, R4, and R5 are each, independently, selected from the group consisting of H, hydroxyl, halogen, and deuterium;
or, alternatively, R2 and R5 together with the carbon atoms to which they are attached, form a single bond;
R3 is selected from the group consisting of H, deuterium, halogen, hydroxyl, and cyano;
or, alternatively, R3 and R1, together with the carbon atoms to which they are attached, form a C3-C5 cycloalkyl;
or, alternatively, R3 and R4, together with the carbon atoms to which they are attached, form a C3-C5 cycloalkyl;
R6, R7, R8, R9, and R11 are each, independently, selected from the group consisting of H, hydroxyl, halogen, and deuterium;
each R12 and R13 is, independently, selected from the group consisting of H, halogen, deuterium, unsubstituted C1-C3 alkyl, and C1-C3 alkyl substituted with hydroxyl or one or more halogen; and
R14, R15, and R16 are each, independently, selected from the group consisting of H, unsubstituted C1-C3 alkyl or C1-C3 alkyl substituted with one or more halogen; and
each R17 and R18 is, independently, selected from the group consisting of H, unsubstituted C1-C3 alkyl or C1-C3 alkyl substituted with one or more halogen;
with the proviso that one or more of (a)-(f) is present:
(a) at least one R is selected from the group consisting of hydroxyl, cyano, unsubstituted C1-C3 alkyl, and C1-C3 alkyl substituted with one or more halogen or deuterium;
(b) E is NRaRb, C1-C3 alkylene-NRaRb, C2-C3 alkyl, C2-C4 alkenyl, C2-C4 alkynyl, C3-C8 cycloalkyl, C1-C3 alkylene-(C3-C8 cycloalkyl), 4- to 10-membered heterocyclyl, C1-C3 alkylene-(4- to 10-membered heterocyclyl), C6-C10 aryl, C1-C3 alkylene-(C6-C10 aryl), 5- to 10-membered heteroaryl, or C1-C3 alkylene-(5- to 10-membered heteroaryl), wherein the C1-C3 alkylene-NRaRb, C2-C3 alkyl, C2-C4 alkenyl, C2-C4 alkynyl, C3-C8 cycloalkyl, C1-C3 alkylene-(C3-C8 cycloalkyl), 4- to 10-membered heterocyclyl, C1-C3 alkylene-(4- to 10-membered heterocyclyl), C6-C10 aryl, C1-C3 alkylene-(C6-C10 aryl), 5- to 10-membered heteroaryl, or C1-C3 alkylene-(5- to 10-membered heteroaryl) is unsubstituted or substituted with one or more halogen, hydroxyl, C1-C3 alkyl, or C1-C3 alkoxyl;
(c) E is C1 alkyl substituted with one or more halogen, hydroxyl, C1-C3 alkyl, or C1-C3 alkoxyl;
(d) at least one of R14, R15, R16, R17, and R18 is unsubstituted C1-C3 alkyl or C1-C3 alkyl substituted with one or more halogen;
(e) at least one of R1, R2, R4, R5, R6, R7, R8, R9, and Ru is hydroxyl; or
(f) at least one of R12 and R13 is C1-C3 alkyl substituted with hydroxyl.
|