| CPC C07C 209/62 (2013.01) [C07C 209/86 (2013.01); C07F 9/222 (2013.01); C07F 9/564 (2013.01); G01N 33/946 (2013.01)] | 20 Claims |
|
1. A process of making levoamphetamine sulfate, said process comprising:
preparing an in situ phenyl magnesium bromide from a reaction of bromobenzene with magnesium in the presence of catalytic diisobutyl aluminum hydride (DIBAL) in tetrahydrofuran (THF) refluxing at a temperature of 60 deg Celsius;
reacting a compound of Formula 4
 with the in situ phenyl magnesium bromide and a copper catalyst under solvent and temperature conditions effective to produce a compound of Formula 5 having a regioisomeric purity >99%:
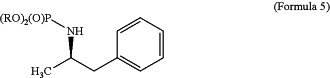 wherein R is alkyl or aryl; and
deprotecting the compound of Formula 5 at a temperature of 80 deg Celsius and under acidic conditions effective to produce levoamphetamine free base
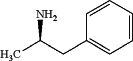 and then levoamphetamine sulfate of Formula I:
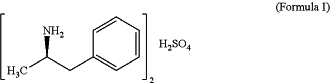 wherein the solvent conditions for preparing the compound of Formula 5 comprise a crystallization step requiring a mixture of two or more solvents, wherein one of the two or more solvents is residue THF.
|