| CPC A61L 31/14 (2013.01) [A61K 9/0087 (2013.01); A61K 9/025 (2013.01); A61K 9/2072 (2013.01); A61K 9/4808 (2013.01); A61K 9/703 (2013.01); A61L 15/34 (2013.01); A61L 15/42 (2013.01); A61L 27/28 (2013.01); A61L 27/34 (2013.01); A61L 27/50 (2013.01); A61L 28/0034 (2013.01); A61L 28/0061 (2013.01); A61L 28/0069 (2013.01); A61L 29/08 (2013.01); A61L 29/085 (2013.01); A61L 29/14 (2013.01); A61L 31/08 (2013.01); A61L 31/10 (2013.01); A61L 2400/10 (2013.01); A61L 2400/12 (2013.01); A61L 2400/18 (2013.01); Y10T 428/24355 (2015.01); Y10T 428/24372 (2015.01)] | 29 Claims |
|
1. A medical device or medical implement with high lubricity to flesh or biological fluid and/or inhibited nucleation on its surface, the device or implement comprising:
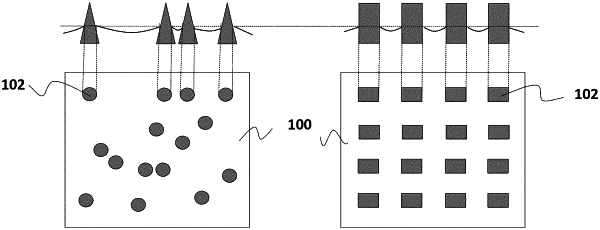
a surface comprising an impregnating liquid and a plurality of micro-scale and/or nano-scale solid features spaced sufficiently close to stably contain the impregnating liquid therebetween,
wherein said impregnating liquid fills spaces between said solid features,
wherein said surface stably contains said impregnating liquid between said solid features,
wherein at least a portion of the micro-scale and/or nano-scale solid features are non-submerged by the impregnating liquid when the surface is in contact with the flesh or the biological fluid,
wherein 0<ϕ<0.3, where ϕ is a surface area fraction of the surface non-submerged by said impregnating liquid,
wherein said surface is a textured surface and said solid features are engineered protrusions or engineered particles of said textured surface, and
wherein the impregnating liquid comprises a medication or drug.
|