| CPC A61K 39/095 (2013.01) [A61K 35/74 (2013.01); A61K 39/0258 (2013.01); A61K 47/6911 (2017.08); A61K 2039/6018 (2013.01); A61K 2039/6087 (2013.01); A61K 2039/627 (2013.01)] | 14 Claims |
|
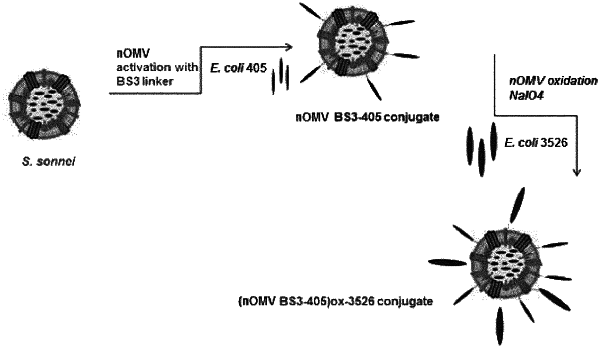
1. An immunogenic conjugate comprising an isolated, intact native outer membrane vesicle (nOMV), having at least a surface saccharide moiety connected to an antigen, and having at least a surface protein residue connected to a different antigen through a bivalent linker;
wherein said bivalent linker has the general formula (I):
X-L-X′ (I)
wherein:
X and X′ are different to each other or the same, and are a functional group able to selectively react with the nOMV protein residue on one hand and with the antigen on the other hand;
-L- is a bivalent linear or branched C1-C15 alkyl or alkenyl group optionally substituted, and optionally interrupted by one or more heteroatom selected from:
oxygen (—O—), sulfur (—S—), and nitrogen (NH— or optionally substituted —N— group);
wherein X and X′ are N-hydroxysuccinimide ester derivatives selected from at least one of:
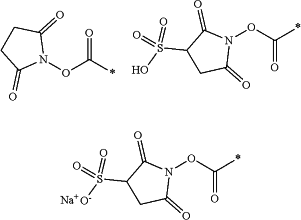 wherein the * represents the point of contact with the -L- group in formula (I);
wherein said nOMV is prepared from S. sonnei, S. flexneri, meningococcus, or Salmonella bacteria;
wherein said antigen is selected from a group consisting of: Neisseria meningitidis fHbp, SsIE, and FdeC;
wherein SsIE comprise an amino acid sequence having 90%, 91%, 92%, 93%, 94%, 95%, 96%, 97%, 98%, 99%, or 99.5% identity to SEQ ID NO: 15;
wherein FdeC comprise an amino acid sequence having 90%, 91%, 92%, 93%, 94%, 95%, 96%, 97%, 98%, 99%, or 99.5% identity to SEQ ID NO: 16; and
wherein Neisseria meningitidis fHbp comprise an amino acid sequence comprising SEQ ID NO: 8, SEQ ID NO: 1, or of SEQ ID NO: 9.
|