| CPC A61K 31/593 (2013.01) [A61K 31/519 (2013.01); A61K 45/06 (2013.01); A61P 17/06 (2018.01)] | 21 Claims |
|
1. A pharmaceutical formulation for topical treatment of a skin disease, comprising (a) a JAK inhibitor, or a pharmaceutically acceptable salt thereof, and (b) a vitamin D3 analog, or a pharmaceutically acceptable salt thereof, wherein the JAK inhibitor, or a pharmaceutically acceptable salt thereof, is a JAK1/2 inhibitor, which is ruxolitinib, or a pharmaceutically acceptable salt thereof; and the vitamin D3 analog, or a pharmaceutically acceptable salt thereof, is a compound of Formula (II):
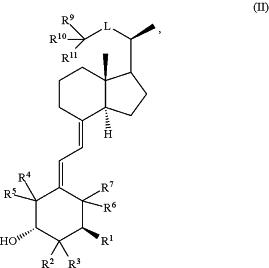 wherein:
R1 is H or OH;
R2 and R3 are each H; or
R2 is O—R2A; and R3 is H; or
R2 and R3 are taken together to form a ═CH2 group;
R2A is —C1-4 alkylene-OH;
R4 and R5 are each H; or
R4 and R5 are taken together to form a ═CH2 group;
R6 and R7 are each H; or
R6 and R7 are taken together to form a ═CH2 group;
L is —CH2—CH2—CH(R12)—, —CH2—CH2—CH2—CH(R12)—, —CH═CH—CH(R12)—, —CH═CH—CH═CH—, —CH2—C≡C—, —O—CH2—CH2—, or —O—CH2—CH2—CH2—, wherein R12 is H or OH;
R9 is C1-3 alkyl or C1-4 haloalkyl;
R10 is C1-3 alkyl or C1-4 haloalkyl;
R11 is H or OH;
or, alternatively, R9 and R10 together with the carbon atom to which they are attached form a C3-4 cycloalkyl ring; and
R11 is H.
|