| CPC A61K 31/428 (2013.01) [A61K 9/006 (2013.01); A61K 9/1617 (2013.01); A61K 9/2004 (2013.01); A61K 31/454 (2013.01); A61K 31/496 (2013.01); A61K 31/506 (2013.01); A61K 31/5377 (2013.01); A61K 31/541 (2013.01); A61K 38/05 (2013.01); A61K 38/06 (2013.01); A61K 39/4636 (2023.05); A61K 45/06 (2013.01); A61P 25/00 (2018.01); A61P 25/28 (2018.01); A61P 35/00 (2018.01); C07D 277/82 (2013.01); C07D 417/12 (2013.01); C07K 5/06026 (2013.01); C07K 5/0806 (2013.01); C07K 5/0808 (2013.01); C07K 5/0812 (2013.01); A61K 2300/00 (2013.01)] | 6 Claims |
|
1. A method for treating spinocerebellar ataxia, comprising administering to a subject in need of such treatment an effective amount of at least one compound having formula:
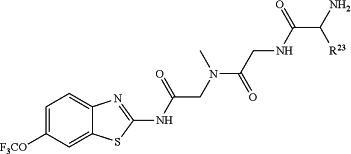 wherein R23 is selected from the group consisting of H, CH3, CH2CH3, CH2CH2CH3, CH2CCH, CH(CH3)2, CH2CH(CH3)2, CH(CH3)CH2CH3, CH2OH, CH2OCH2Ph, CH2CH2OCH2Ph, CH(OH)CH3, CH2Ph, CH2(cyclohexyl), CH2(4-OH-Ph), (CH2)4NH2, (CH2)3NHC(NH2)NH, CH2(3-indole), CH2(5-imidazole), CH2CO2H, CH2CH2CO2H, CH2CONH2, and CH2CH2CONH2; or an enantiomer, diastereomer, hydrate, solvate, pharmaceutically acceptable salt, or complex thereof.
|