| CPC A61F 9/0017 (2013.01) [A61F 9/00781 (2013.01); A61K 9/0051 (2013.01); A61K 31/559 (2013.01); A61K 47/34 (2013.01); C08L 67/04 (2013.01); A61F 2210/0004 (2013.01)] | 6 Claims |
|
1. A biodegradable intraocular implant comprising about 15% by weight of Compound 1:
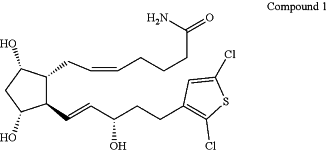 about 5% by weight of a first polymer that is poly(D,L-lactide) having an acid end group and an inherent viscosity of about 0.16-0.24 dl/g, about 30% by weight of a second polymer that is poly(D,L-lactide) having an ester end group and an inherent viscosity of about 0.25-0.35 dl/g, about 45% by weight of a third polymer that is poly(D,L-lactide-co-glycolide) having an ester end group and an inherent viscosity of about 0.16-0.24 dl/g and a D,L-lactide:glycolide ratio of about 75:25, about 3% by weight cetyl alcohol, and about 2% by weight butylated hydroxyanisole, wherein the inherent viscosities of the first, second, and third polymers correspond to those measured for a 0.1% solution of the polymer in chloroform at 25° C.;
and wherein the implant releases in vitro less than 30% of Compound 1 during the first 24 hours wherein the in vitro release of Compound 1 is measured in a phosphate buffered saline (PBS) solution at a pH of 7.4±0.05 and at 37° C., and wherein the PBS solution is a PBS solution that is free of magnesium and calcium and has a pH of 7.4±0.05 at 25° C.
|