| CPC A61F 2/95 (2013.01) [A61F 2/07 (2013.01); A61F 2/958 (2013.01); A61F 2002/072 (2013.01); A61F 2002/075 (2013.01); A61F 2/243 (2013.01); A61F 2/848 (2013.01); A61F 2002/8483 (2013.01); A61F 2/89 (2013.01); A61F 2002/91591 (2013.01); A61F 2002/9505 (2013.01); A61F 2002/9511 (2013.01); A61F 2210/0014 (2013.01); A61F 2210/0076 (2013.01); A61F 2220/0016 (2013.01); A61F 2220/005 (2013.01); A61F 2220/0058 (2013.01); A61F 2230/005 (2013.01); A61F 2230/0054 (2013.01); A61F 2250/0003 (2013.01); A61F 2250/0098 (2013.01); A61L 31/022 (2013.01)] | 20 Claims |
|
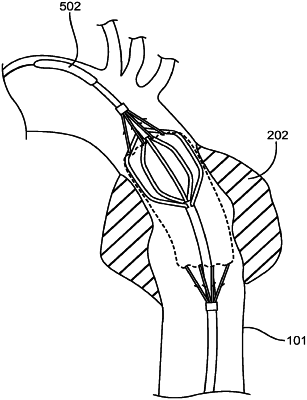
1. A delivery system for delivering a stent graft, comprising:
a delivery catheter comprising an elongate shaft;
a stent graft having a stent portion and a main graft body with an inner lumen, the stent graft being loaded on a section of the delivery catheter such that the elongate shaft is disposed within the inner lumen;
an expandable device having an expandable portion, the expandable portion disposed on the elongate shaft within the inner lumen and configured to fully radially expand within the inner lumen to radially expand the main graft body; and
a constraining device configured to radially constrain the stent portion in a radially constrained state while the main graft body is radially expanded, and
wherein the expandable device is configured to be expanded prior to deployment of the stent portion of the stent graft.
|