| CPC C22C 13/00 (2013.01) [B23K 35/262 (2013.01); C22B 11/00 (2013.01); C22B 13/08 (2013.01); C22B 19/32 (2013.01); C22B 25/04 (2013.01); C22B 25/08 (2013.01); C22B 58/00 (2013.01); C25C 1/12 (2013.01); C25C 1/14 (2013.01); C25C 1/16 (2013.01); C25C 1/20 (2013.01); C25C 1/22 (2013.01); B23K 2101/40 (2018.08); C22C 2202/00 (2013.01)] | 5 Claims |
|
5. A method for producing a tin alloy having a low α-ray emission, the method including the step of producing the tin alloy having a low α-ray emission by adding one or more metals selected from a group consisting of silver, copper, zinc, and indium to metallic tin and mixing thereof to obtain a mixture, and casting the mixture,
wherein each of the metallic tin and the one or more metals added to the metallic tin are produced using a method including:
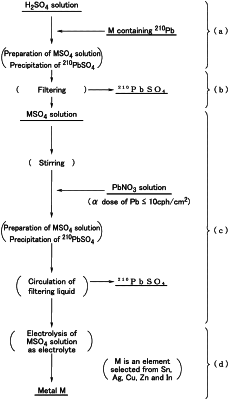
a step (a) of dissolving any metal of tin, silver, copper, zinc and indium, each including lead as an impurity in a sulfuric acid aqueous solution, to prepare a hydrosulfate aqueous solution of the metal and performing a first precipitation of lead sulfate by precipitating lead sulfate in the hydro sulfate aqueous solution;
a step (b) of filtering the hydrosulfate aqueous solution obtained from the step (a) to remove the lead sulfate from the hydrosulfate aqueous solution;
a step (c) of performing a second precipitation of lead sulfate in the hydrosulfate aqueous solution by adding a lead nitrate aqueous solution of a predetermined concentration including lead having an α-ray emission of 10 cph/cm2 or less to the hydrosulfate aqueous solution obtained from the step (b), from which lead sulfate has already been removed, at a predetermined addition rate for 30 minutes or longer while stirring the hydrosulfate aqueous solution at a rotation rate of at least 100 rpm in a first tank, and, at the same time, circulating the hydrosulfate aqueous solution in the first tank at a proportion at which a circulation flow rate reaches at least 1% by volume of a total liquid amount while filtering the hydrosulfate aqueous solution to remove lead sulfate which has precipitated from the hydrosulfate aqueous solution a second time; and
a step (d) of transferring the hydrosulfate aqueous solution obtained from the step (c) to a separate second tank from the first tank and then electrowinning the metal using the hydrosulfate aqueous solution obtained from step (c) as an electrolytic solution.
|