| CPC C22B 5/04 (2013.01) [C21D 1/74 (2013.01); C21D 6/002 (2013.01); C21D 8/1205 (2013.01); C21D 9/0025 (2013.01); C21D 9/0068 (2013.01); C22B 1/24 (2013.01); C22C 33/04 (2013.01); C22C 38/18 (2013.01)] | 7 Claims |
|
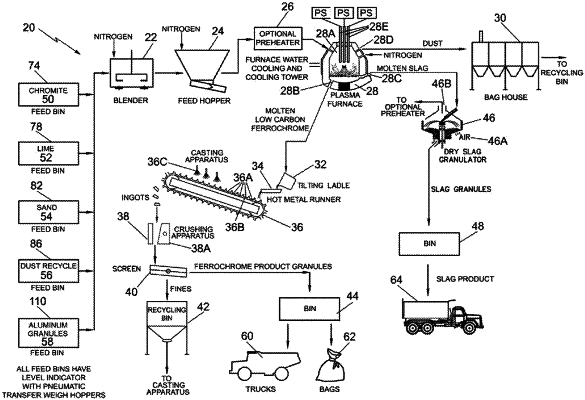
1. A method for recovering a ferroalloy from chromite ore comprising:
feeding a mixture of feed materials comprising chromite ore and aluminum granules into a plasma arc furnace, said chromite ore including chromium oxide and an iron oxide, said feed materials being in a proportion for reduction of said chromium oxide and iron oxide to form said ferroalloy;
heating said feed materials in said plasma arc furnace to a temperature in the range of approximately 1,650° C. to 1850° C. wherein said aluminum granules act as a reducing agent to produce an exothermic reaction reducing said chromium oxide and iron oxide in said chromite ore to produce a molten ferroalloy with molten slag floating on top of said molten ferroalloy;
extracting said molten ferroalloy from said plasma arc furnace; and
wherein the amount of aluminum granules used in said mixture of feed materials is equivalent to approximately 105% to 120% of the stoichiometric quantity of aluminum required to react with said chromite ore in said mixture of feed materials.
|