| CPC C12Q 1/6869 (2013.01) [C07H 19/10 (2013.01); C07H 19/14 (2013.01); C07H 19/20 (2013.01)] | 21 Claims |
|
1. A compound of the formula:
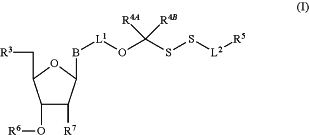 Wherein
B is a base;
L1 is covalent linker;
L2 is covalent linker;
R3 is —OH, monophosphate, polyphosphate or a nucleic acid;
R4A is hydrogen, —CH3, —CX13, —CHX12, —CH2X1, —CN, —Ph, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl;
R4B is hydrogen, —CH3, —CX23, —CHX22, —CH2X2, —CN, —Ph, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted aryl, or substituted or unsubstituted heteroaryl;
R5 is a detectable label or anchor moiety;
R6 is hydrogen or a polymerase-compatible cleavable moiety and the polymerase-compatible cleavable moiety is —CH2—CH═CH2, —CH2N3 or
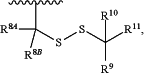 R8A is hydrogen;
R8B is hydrogen;
R9, R10, and R11 are each independently hydrogen or unsubstituted alkyl;
R7 is hydrogen or —OR7A, wherein R7A is hydrogen; and
X1 and X2 are independently halogen.
|