| CPC C12Q 1/6827 (2013.01) [G16H 50/30 (2018.01); C12Q 2600/106 (2013.01); C12Q 2600/112 (2013.01); C12Q 2600/118 (2013.01)] | 8 Claims |
|
1. A method of treating a non-small cell lung cancer (NSCLC) in a human patient, the method comprising:
administering a protein arginine N-methyltransferase 5 (PRMT5) inhibitor to the human patient, wherein the human patient has been determined to have a I696fs mutation in RBM10 wherein the PRMT5 inhibitor is
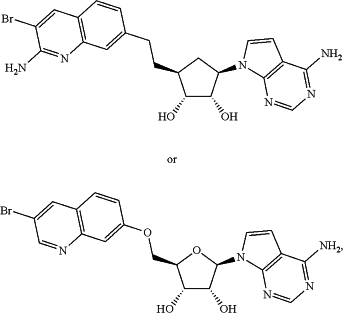 or a pharmaceutically acceptable salt thereof or a solvate thereof.
|