| CPC C08G 71/04 (2013.01) [C07D 405/14 (2013.01); C07D 407/14 (2013.01); C08F 283/00 (2013.01); C09D 175/12 (2013.01)] | 17 Claims |
|
1. A compound represented by general formula (Ib):
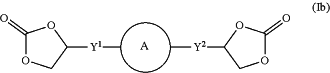 wherein
ring A is selected from the group consisting of thiophene, pyrrole, oxazole, isoxazole, isothiazole, tetrahydrofuran, tetrahydrothiophene, pyrrolidine, pyridine, pyrone, pyridazine, pyrimidine, pyrazine, triazine, piperidine and piperazine;
Y1 and Y2 are each independently selected from the group consisting of:
a single bond,
—Z—O—Z—,
—Z—NRb—Z—,
—Z—O—C(═O)—Z—, —Z—C(═O)—O—Z—,
—Z—NRb—C(═O)—Z—, —Z—C(═O)—NRb—Z—,
—Z—NRb—C(═O)—O—Z, Z—O—C(═O)—NRb—Z—;
where each Z is independently selected from the group consisting of a single bond, optionally substituted saturated aliphatic chain and optionally substituted unsaturated aliphatic chain; and
where Rb is H or C1-C6 alkyl.
|