| CPC C07D 487/04 (2013.01) | 11 Claims |
|
1. A process for the synthesis of 6-((3S,4S)-4-methyl-1-(pyrimidin-2-ylmethyl)pyrrolidin-3-yl)-3-(tetrahydropyran-4-yl-7H-imidazo[1,5-α]pyrazin-8-one (Compound P3.1) of the following formula:
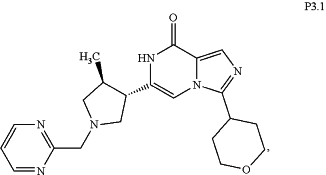 wherein the process comprises the following steps:
(a) reacting a compound of formula (S,S)-4:
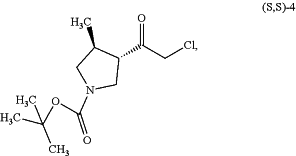 with a compound of formula 9:
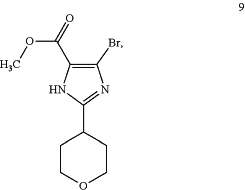 in the presence of potassium carbonate (K2CO3), followed by reacting with ammonium acetate (NH4OAc), to provide a compound of formula (S,S)-10:
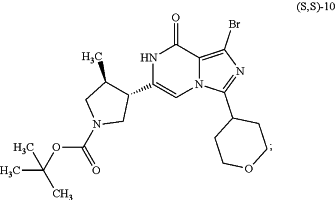 (b) reacting the compound of formula (S,S)-10 above with hydrogen gas in the presence of a Pd/C catalyst, to provide a compound of formula (S,S)-11:
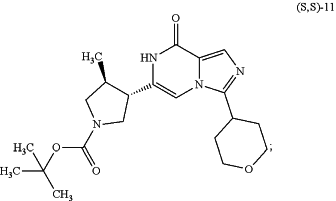 (c) reacting the compound of formula (S,S)-11 above with hydrochloric acid (HCl), to provide a compound of formula (S,S)-12:
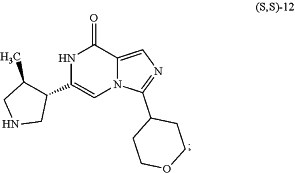 and
(d) reacting the compound of formula (S,S)-12 above with a compound of the following formula:
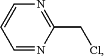 in the presence of N,N-diisopropylethylamine (DIEA), to provide 6-((3S,4S)-4-methyl-1-(pyrimidin-2-ylmethyl)pyrrolidin-3-yl)-3-(tetrahydropyran-4-yl-7H-imidazo[1,5-α]pyrazin-8-one (Compound P3.1) above;
wherein the process does not comprise chromatographic chiral resolution of the enantiomeric forms of any intermediates; and
wherein the process does not comprise chromatographic chiral resolution of the enantiomeric forms of 6-((3S,4S)-4-methyl-1-(pyrimidin-2-ylmethyppyrrolidin-3-yl)-3-(tetrahydropyran-4-yl-7H-imidazo [1,5-α]pyrazin-8-one (Compound P3.1).
|