| CPC C07D 231/14 (2013.01) [C07D 401/12 (2013.01); C07D 403/12 (2013.01); C07D 405/06 (2013.01); C07D 405/14 (2013.01); C07D 413/12 (2013.01)] | 16 Claims |
|
1. A compound according to general formula (I)
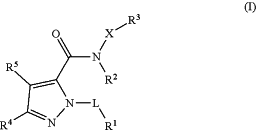 wherein
L represents CH2, CH(CH3) or CH(CH2CH3);
R1 represents C3-10-cycloalkyl, or 4 to 10-membered heterocycloalkyl,
R2 represents H or C1-6-alkyl;
X represents phenyl, or 5 to 10-membered heteroaryl;
R3 represents S(O)2—C1-6-alkyl, S(O)2—(C3-6-cycloalkyl), S(O)2-(4 to 6-membered heterocycloalkyl), S(O)2-phenyl, S(O)2-(5 or 6-membered heteroaryl), S(O)—NH2, S(O)—N(H)(C1-6-alkyl), S(O)—N(C1-6-alkyl)2, S(O)2—NH2, S(O)2—N(H)(C1-6-alkyl), S(O)2—N(H)(C3-6-cycloalkyl), S(O)2—N(C1-6-alkyl)2, C(O)—NH2, C(O)—N(H)(C1-6-alkyl), C(O)—N(H)(C3-6-cycloalkyl), C(O)—N(C1-6-alkyl)2, C(O)—N(C1-6-alkyl)(C3-6-cycloalkyl), OCF3, OCF2H, CN, OH, O—C3-6-cycloalkyl, O-(4 to 6-membered heterocycloalkyl), S(O)—C1-6-alkyl, S(O)—(C3-6-cycloalkyl), S(O)-(4 to 6-membered heterocycloalkyl), S(O)-phenyl, or S(O)-(5 or 6-membered heteroaryl);
R4 and R5 independently from one another represent H, F, Cl, Br, CN, CHF2, CH2F, CF3, C1-6-alkyl, C3-10-cycloalkyl, C1-6-alkylene-C3-10-cycloalkyl, 4 to 10-membered heterocycloalkyl, C1-6-alkylene-(4 to 10-membered heterocycloalkyl), NH2, N(H)(C1-6-alkyl), N(C1-6-alkyl)2, O—C1-6-alkyl, O—C3-10-cycloalkyl, O—C1-6-alkylene-C3-10-cycloalkyl, O-(4 to 6-membered heterocycloalkyl), or O—C1-6-alkylene-(4 to 6-membered heterocycloalkyl), provided that at least one of R4 and R5 does not represent H;
wherein C1-6-alkyl and C1-6-alkylene in each case independently from one another is linear or branched;
wherein C1-6-alkyl, C1-6-alkylene, C3-10-cycloalkyl, C3-6-cycloalkyl, 4 to 10-membered heterocycloalkyl and 4 to 6-membered heterocycloalkyl in each case independently from one another are unsubstituted or mono- or polysubstituted with F; and/or are unsubstituted or monosubstituted with one substituent selected from the group consisting of Cl, CN, C1-6-alkyl, C1-6-alkylene-OH, C1-6-alkylene-OCH3, CF3, CF2H, CFH2, C(O)—C1-6-alkyl, OH, ═O, OCF3, OCF2H, OCFH2, O—C1-6-alkyl, C1-4-alkylene-O—C1-4-alkylene-O—CH3, and C0-4-alkylene-O—(C1-4-alkylene-O)1-4—CH3;
wherein phenyl, 5 to 10-membered heteroaryl and 5 or 6-membered heteroaryl in each case independently from one another are unsubstituted or mono- or polysubstituted with one or more substituents selected from the group consisting of F, Cl, Br, CN, C1-6-alkyl, C3-6-cycloalkyl, CF3, CF2H, CFH2, C1-6-alkylene-CF3, C1-6-alkylene-CF2H, C1-6-alkylene-CFH2, C1-6-alkylene-OH, C1-6-alkylene-OCH3, C(O)—C1-6-alkyl, OCF3, OCF2H, OCFH2, and O—C1-6-alkyl;
in the form of the free compound or a physiologically acceptable salt thereof.
|