| CPC A61M 16/0051 (2013.01) [A61M 16/024 (2017.08); A61M 16/1005 (2014.02); A61M 16/12 (2013.01); A61M 16/122 (2014.02); A61M 16/125 (2014.02); A61M 16/201 (2014.02); A61M 16/202 (2014.02); A61M 16/204 (2014.02); B63C 11/02 (2013.01); G01F 22/02 (2013.01); A61M 2016/0027 (2013.01); A61M 2016/0039 (2013.01); A61M 16/085 (2014.02); A61M 2016/102 (2013.01); A61M 16/208 (2013.01); A61M 2202/0275 (2013.01); A61M 2205/14 (2013.01); A61M 2205/16 (2013.01); A61M 2205/17 (2013.01); A61M 2205/3331 (2013.01); A61M 2205/3334 (2013.01); A61M 2205/3368 (2013.01); A61M 2205/3379 (2013.01); A61M 2205/50 (2013.01); A61M 2205/502 (2013.01); A61M 2205/52 (2013.01); A61M 2205/60 (2013.01); A61M 2205/6018 (2013.01); A61M 2205/6072 (2013.01); A61M 2205/8206 (2013.01); F17C 2250/00 (2013.01)] | 21 Claims |
|
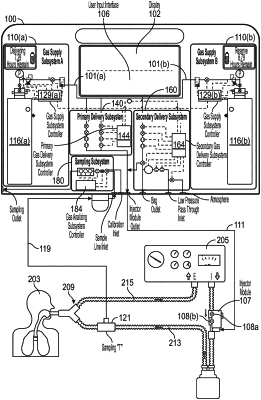
1. A therapeutic gas delivery system for confirming the proper functioning of gas delivery and injection module operation, comprising:
an inlet port;
a low pressure outlet port in fluid communication with the inlet port;
an injection module operable to be received at the low pressure outlet port;
a low pressure gas supply operable to provide a flow of breathing gas at the inlet port, wherein the flow of breathing gas is at a breathing gas flow rate;
a low pressure delivery flow sensor and a low pressure confirmatory flow sensor operable to measure the breathing gas flow rate from the low pressure gas supply, wherein the low pressure delivery flow sensor and the low pressure confirmatory flow sensor are in fluid communication with the inlet port and the low pressure outlet port;
an injector module having an injection module delivery flow sensor and an injection module confirmatory flow sensor operable to measure the breathing gas flow rate from the low pressure gas supply, wherein the injection module delivery flow sensor and the injection module confirmatory flow sensor are in fluid communication with the low pressure outlet port; and
a controller operable to determine if one of the measured breathing gas flow rates differs from any one of the other measured breathing gas flow rates by greater than a threshold amount, wherein the flow rate is measured at two or more of the low pressure confirmatory flow sensor, the low pressure delivery flow sensor, the injection module confirmatory flow sensor, and/or the injection module delivery flow sensor.
|