| CPC A61K 9/06 (2013.01) [A61K 31/44 (2013.01); A61K 47/10 (2013.01); A61K 47/26 (2013.01); A61K 47/32 (2013.01); A61K 47/38 (2013.01); A61K 9/0014 (2013.01)] | 5 Claims |
|
1. A topical sol-gel composition, comprising
a first component comprising microcrystalline cellulose and carboxymethylcellulose sodium, wherein the first component is contained in an amount of 1 to 10 wt. %, based on the total weight of the topical sol-gel composition;
a second component that is any one selected from the group consisting of hydroxypropyl methylcellulose (HPMC), polyvinylpyrrolidone vinyl acetate (PVP VA), and a mixture thereof; and
a third component that is any one selected from the group consisting of poloxamer, polysorbate, and a mixture thereof, wherein the third component is contained in an amount of 0.5 to 5 wt. % based on a total weight of the sol-gel composition;
wherein the topical sol-gel composition comprises a polyol,
wherein the topical sol-gel composition comprises a drug, wherein the drug is (R)—N-[1-(3,5-difluoro-4-methansulfonylamino-phenyl)-ethyl]-3-(2-propyl-6-trifluoromethyl-pyridin-3-yl)-acrylamide, and
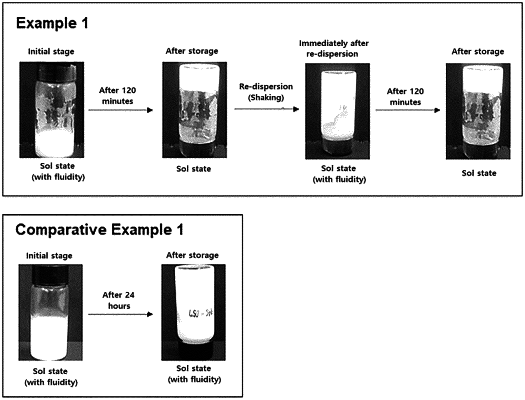
wherein the topical sol gel composition is a reversible sol gel composition that forms a sol state by application of external physical force, and has a viscosity of 50,000 cps in the gel state and a viscosity of 5,000 cps in the sol state, wherein it takes about 110 min to 120 min for formation of the gel state from the sol state when the sol state is left without application of an external physical force.
|