| CPC A61K 47/65 (2017.08) [A61K 38/15 (2013.01); A61K 47/10 (2013.01); A61K 47/22 (2013.01); A61K 47/64 (2017.08); A61K 47/6829 (2017.08); A61K 47/6889 (2017.08); A61P 35/00 (2018.01); C07D 405/06 (2013.01); C07D 405/14 (2013.01); C07K 11/00 (2013.01)] | 17 Claims |
|
1. A compound of formula (V):
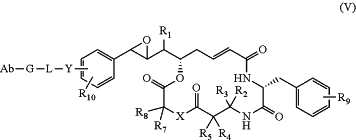 wherein:
R1 is (C1-C6)alkyl;
R2 is hydrogen or (C1-C6)alkyl;
R3 is hydrogen or (C1-C6)alkyl;
R4 is hydrogen, (C1-C6)alkyl, (C1-C6)alkylene-C(O)OH, (C1-C6)alkylene-NHR12, (C1-C6)alkylene-OH, or (C1-C6)alkylene-SH;
R5 is hydrogen, (C1-C6)alkyl, (C1-C6)alkylene-C(O)OH, (C1-C6)alkylene-NHR12, (C1-C6)alkylene-OH, or (C1-C6)alkylene-SH;
X is —NR6— or —O—;
R6 is hydrogen or (C1-C6)alkyl;
R7 is hydrogen, (C1-C6)alkyl, (C1-C6)alkylene-C(O)OH, or (C1-C6)alkylene-N[(C1-C6)alkyl]2;
R8 is hydrogen, (C1-C6)alkyl, (C1-C6)alkylene-C(O)OH, or (C1-C6)alkylene-N[(C1-C6)alkyl]2;
each R9 is independently hydrogen, halogen, NH2, NH(C1-C6)alkyl, N[(C1-C6)alkyl]2, NH(C3-C6)cycloalkyl, OH, O(C1-C4)alkyl, or (C3-C6)heterocycloalkyl;
each R10 is independently hydrogen or (C1-C4)alkyl;
each R12 is independently hydrogen or (C1-C6)alkyl;
Y is —NR11—(C1-C6)alkylene-, —O—(C1-C6)alkylene-, or —S—(C1-C6)alkylene-, wherein the (C1-C6)alkylene of Y is bonded to the phenyl ring and the —NR11—, —O—, or —S— of Y is either (a) bonded directly to the —C(O)— of L1 in formula (II) or (b) bonded directly to the —C(O)— of an (AA)w of L1 in formula (II);
R11 is hydrogen or (C1-C6)alkyl; and
L is formula (II):
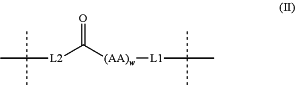 wherein:
L1 is formula (III):
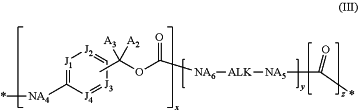 wherein:
(i) x is 0;
y is 0; and
z is 0; or
(ii) x is 1;
y is 0; and
z is 0; or
(iii) x is 1;
y is 1; and
z is 1;
A5 is hydrogen or (C1-C6)alkyl;
ALK is (C1-C12)alkylene;
A6 is hydrogen or (C1-C6)alkyl;
A2 is hydrogen or (C1-C6)alkyl;
A3 is hydrogen or (C1-C6)alkyl;
J1 is CA1 or N;
J2 is CA1 or N;
J3 is CA1 or N;
J4 is CA1 or N;
each A1 is independently hydrogen or (C1-C6)alkyl; and
A4 is hydrogen or (C1-C6)alkyl;
wherein L1 is bonded directly to —O—, or —S— of Y;
(AA)w is a sequence of w amino acids connected together via peptide bonds, wherein each amino acid, (AA) W, is independently a natural or non-natural amino acid, (AAns);
w is 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, or 12;
each (AA n O is independently selected from the group consisting of alanine (Ala), γ-aminobutyric acid (GABA), arginine (Arg), asparagine (Asn), aspartic acid (Asp), citrulline (Cit), cysteine (Cys), glutamine (Gln), glutamic acid (Glu), glycine (Gly), histidine (His), isoleucine (Ile), leucine (Leu), lysine (Lys), ε-(acetyl)-lysine ((ε-Ac)Lys), methionine (Met), phenylalanine (Phe), proline (Pro), serine (Ser), threonine (Thr), tryptophan (Trp), tyrosine (Tyr), and valine (Val); and
L2 is:
(a) —(C1-C6)alkylene-NA7-C(O)—(C1-C6)alkylene-;
(b) —C(O)—(C1-C6)alkylene-NA7-C(O)—(C1-C6)alkylene-;
(c) —NA8-(C1-C6)alkylene-NA7-C(O)—(C1-C6)alkylene-;
(d) —C(O)—NA8-(C1-C6)alkylene-NA7-C(O)—(C1-C6)alkylene-;
(e) —NA7-(C1-C6)alkylene-;
(f) —(C1-C6)alkylene-NA7-(C1-C6)alkylene-;
(g) —C(O)—(C1-C6)alkylene-NA7-(C1-C6)alkylene-;
(h) —NA8-(C1-C6)alkylene-NA7-(C1-C6)alkylene-;
(i) —C(O)—NA8-(C1-C6)alkylene-NA7-(C1-C6)alkylene-;
(j) —C(O)—NA7-(C1-C6)alkylene-;
(k) —(C1-C6)alkylene-C(O)—NA7-(C1-C6)alkylene-;
(l) —NA8-(C1-C6)alkylene-C(O)—NA7-(C1-C6)alkylene-;
(m) —C(O)—NA8-(C1-C6)alkylene-C(O)—NA7-(C1-C6)alkylene-;
(n) —C(O)—(C1-C6)alkylene-C(O)—NA7-(C1-C6)alkylene-;
(o) —(C1-C6)alkylene-(OCH2CH2)i—NA7-(C1-C6)alkylene-;
(p) —C(O)—(C1-C6)alkylene-(OCH2CH2)i—NA7-(C1-C6)alkylene-;
(q) —NA8-(C1-C6)alkylene-(OCH2CH2)i—NA7-(C1-C6)alkylene-;
(r) —C(O)—NA8-(C1-C6)alkylene-(OCH2CH2)i—NA7-(C1-C6)alkylene-;
(s) —C(O)—NA7-(OCH2CH2)i—(C1-C6)alkylene-;
(t) —NA7-(CH2CH2O)i—(C1-C6)alkylene-;
(u) —(C1-C6)alkylene-NA7-;
(v) —C(O)—(C1-C6)alkylene-NA7-;
(w) —NA8-(C1-C6)alkylene-NA7-;
(x) —C(O)—NA8-(C1-C6)alkylene-NA7-;
(y) —(C1-C6)alkylene-NA7-C(O)—(C1-C6)alkylene-(OCH2CH2)i—;
(z) —C(O)—(C1-C6)alkylene-NA7-C(O)—(C1-C6)alkylene-(OCH2CH2)i—;
(aa) —NA8-(C1-C6)alkylene-NA7-C(O)—(C1-C6)alkylene-(OCH2CH2)i—;
(bb) —C(O)—NA8-(C1-C6)alkylene-NA7-C(O)—(C1-C6)alkylene-(OCH2CH2)i—;
(cc) —(C1-C6)alkylene-NA7-(C1-C6)alkylene-(OCH2CH2)i—;
(dd) —C(O)—(C1-C6)alkylene-NA7-(C1-C6)alkylene-(OCH2CH2)i—;
(ee) —NA8-(C1-C6)alkylene-NA7-(C1-C6)alkylene-(OCH2CH2)i—;
(ff) —C(O)—NA8-(C1-C6)alkylene-NA7-(C1-C6)alkylene-(OCH2CH2)i—;
(gg) —(C1-C6)alkylene-C(O)—NA7-(C1-C6)alkylene-(OCH2CH2)i—;
(hh) —C(O)—(C1-C6)alkylene-C(O)—NA7-(C1-C6)alkylene-(OCH2CH2)i—;
(ii) —NA8-(C1-C6)alkylene-C(O)—NA7-(C1-C6)alkylene-(OCH2CH2)i—;
(jj) —C(O)—NA8-(C1-C6)alkylene-C(O)—NA7-(C1-C6)alkylene-(OCH2CH2)i—;
(kk) —NA7-arylene-;
(ll) —C(O)—NA7-arylene-;
(mm) —NA7-heteroarylene-; or
(nn) —C(O)—NA7-heteroarylene-;
A7 is a straight, branched, saturated, or unsaturated C1-C160 hydrocarbon;
wherein one or more —CH2— units of the C1-C160 hydrocarbon are optionally and independently replaced by one or more atoms or groups independently selected from the group consisting of —CH(OH)—, —CH(SO3H)—, —CH(SO3−M+)—, —C(O)—, —C(O)NH—, —C(O)N(alkyl)-, —C(O)O—, —NHC(O)—, —N(alkyl)C(O)—, —NHS(O)2—, —N(alkyl)S(O)2—, —P(O)(OH)—, —P(O)(OH)O—, —O—, —OC(O)—, —OC(O)O—, —OP(O)(OH)—, —OP(O)(OH)O—, —S(O)—, —S(O)2—, —S(O)2NH—, —S(O)2N(alkyl)-, and heterocycloalkyl;
wherein each heterocycloalkyl is optionally and independently substituted by one or more substituents independently selected from the group consisting of halogen, alkyl, NH2, NH(alkyl), N(alkyl)2, OH, and O(alkyl); and
wherein the C1-C160 hydrocarbon is optionally substituted by one or more substituents independently selected from the group consisting of halogen, alkyl, NH2, NH(alkyl), N(alkyl)2, OH, and O(alkyl);
A8 is hydrogen or (C1-C6)alkyl;
M+ is an alkali metal; and
i is 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41,42, 43, 44, 45, 46, 47, 48, 49, or 50;
wherein the right end of L2 is bonded to —C(O)— and the left end of L2 is bonded to RCG1 of G;
G is the product of a reaction between RCG1, a reactive chemical group present at the end of L of formula (II), and RCG2, an orthogonal reactive chemical group present on an antibody, Ab;
(i) RCG1 is:
Cl,C≡CH,NH2,N3,OH,O(alkyl)-NHOH,SH,
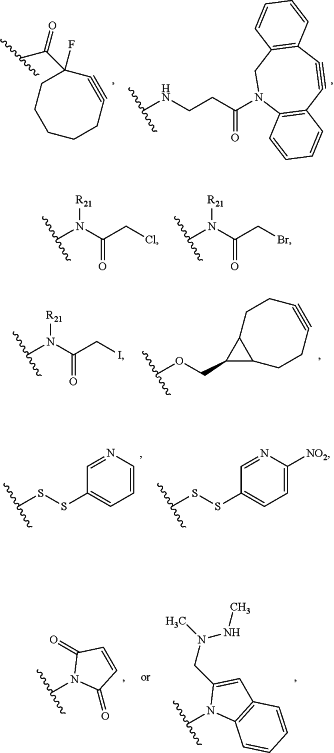 wherein:
R21 is hydrogen or (C1-C6) alkyl; and
the —CO—, —NH—, —NR21—, —N═, —O—, or S is bonded to L2; or
(ii) RCG1 is:
C(O)ZaRa,
wherein:
Za is a single bond, —NH—, or —O—; and
Ra is hydrogen, (C1-C6)alkyl, alkenyl, (C3-C7)cycloalkyl, (C3-C7)heterocycloalkyl, aryl, or heteroaryl, wherein the (C3-C7)heterocycloalkyl, aryl, or heteroaryl is optionally substituted by 1, 2, 3, 4, or 5 substituents independently selected from the group consisting of halogen, CN, NO2, alkyl, OH, O(alkyl), and =0;
wherein —C(O)— is bonded to L2;
RCG2 is:
C≡CH,C(O)H,C(O)C1-C6alkyl,C(O)NH2,C(O)OH,NH2,N3,SH,
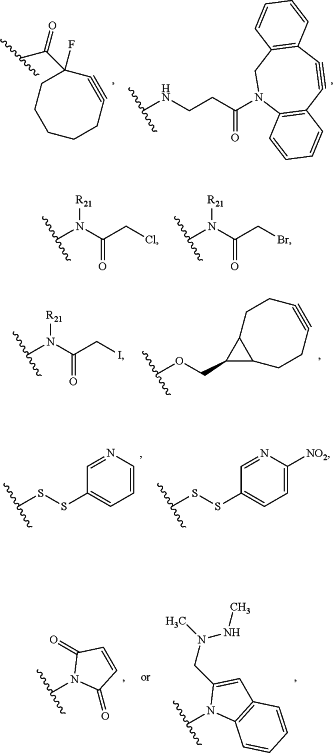 wherein:
R21 is hydrogen or (C1-C6) alkyl; and
Ab is the antibody.
|
|
15. A pharmaceutical composition comprising a compound according to claim 1 and a pharmaceutically acceptable excipient.
|