| CPC A61K 31/519 (2013.01) [A61K 9/12 (2013.01); A61P 11/00 (2018.01); A61P 41/00 (2018.01)] | 3 Claims |
|
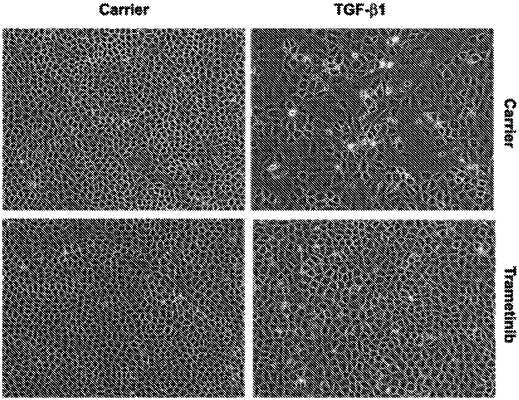
1. A method of reducing the occurrence of excessive fibrin formation in a patient consisting of administering to said patient one effective dose of Trametinib (GSK1120212) sufficient to reduce the fibrin formation prior to a surgical procedure, and a further dose of the Trametinib (GSK1120212) for seven days after said surgical procedure daily, wherein the effective dose of Trametinib (GSK1120212) is between 0.01 mg to 1.5 mg.
|