| CPC A61K 31/496 (2013.01) [A61K 31/444 (2013.01); A61K 31/4985 (2013.01); A61K 31/4995 (2013.01); A61K 31/506 (2013.01); A61K 31/5377 (2013.01); A61K 31/551 (2013.01); A61K 45/06 (2013.01); A61P 1/12 (2018.01); A61P 35/00 (2018.01); A61P 35/02 (2018.01); A61P 35/04 (2018.01); C07D 471/04 (2013.01); C07D 519/00 (2013.01)] | 14 Claims |
|
1. A process for preparing a compound of Formula I:
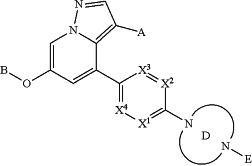 or pharmaceutically acceptable salt or solvate thereof, wherein
X1, X2, X3 and X4 are independently CH, CF, CCH3 or N, wherein zero, one or two of X1, X2, X3 and X4 is N;
A is CN;
B is
(a) hydrogen,
(b) C1-C6 alkyl optionally substituted with 1-3 fluoros,
(c) hydroxyC2-C6 alkyl-, wherein the alkyl portion is optionally substituted with 1-3 fluoros or a C3-C6 cycloalkylidene ring,
(d) dihydroxyC3-C6 alkyl-, wherein the alkyl portion is optionally substituted with a C3-C6 cycloalkylidene ring,
(e) (C1-C6 alkoxy)C1-C6 alkyl- optionally substituted with 1-3 fluoros,
(f) (R1R2N)C1-C6 alkyl- wherein said alkyl portion is optionally substituted with OH and wherein R1 and R2 are independently H or C1-C6 alkyl (optionally substituted with 1-3 fluoros);
(g) hetAr1C1-C3 alkyl-, wherein hetAr1 is a 5-6 membered heteroaryl ring having 1-3 ring heteroatoms independently selected from N, O and S and is optionally substituted with one or more independently selected C1-C6 alkyl substituents;
(h) (C3-C6 cycloalkyl)C1-C3 alkyl-, wherein said cycloalkyl is optionally substituted with OH,
(i) (hetCyca)C1-C3 alkyl-,
(j) hetCyca-,
(k) C3-C6 cycloalkyl-, wherein said cycloalkyl is optionally substituted with OH,
(l) (C1-C4 alkyl)C(═O)O—C1-C6 alkyl-, wherein each of the C1-C4 alkyl and C1-C6 alkyl portions is optionally and independently substituted with 1-3 fluoros, or
(m) (R1R2N)C(═O)C1-C6 alkyl-, wherein R1 and R2 are independently H or C1-C6 alkyl (optionally substituted with 1-3 fluoros);
hetCyca- is a 4-6 membered heterocyclic ring having 1-2 ring heteroatoms independently selected from N and O and optionally substituted with one or more substituents independently selected from OH, C1-C6 alkyl (optionally substituted with 1-3 fluoros), hydroxyC1-C6 alkyl-, C1-C6 alkoxy, (C1-C6 alkyl)C(═O)—, (C1-C6 alkoxy)C1-C6 alkyl-, and fluoro, or wherein hetCyca is substituted with oxo;
Ring D is (i) a saturated 4-7 membered heterocyclic ring having two ring nitrogen atoms, (ii) a saturated 7-8 membered bridged heterocyclic ring having two ring nitrogen atoms and optionally having a third ring heteroatom which is oxygen, (iii) a saturated 7-11 membered heterospirocyclic ring having two ring nitrogen atoms, or (iv) a saturated 9-10 membered bicyclic fused heterocyclic ring having two ring nitrogen atoms, wherein each of said rings is optionally substituted with (a) one to four groups independently selected from halogen, OH, C1-C3 alkyl which is optionally substituted with 1-3 fluoros, or C1-C3 alkoxy which is optionally substituted with 1-3 fluoros, (b) a C3-C6 cycloalkylidene ring, or (c) an oxo group;
E is
(a) hydrogen,
(b) C1-C6 alkyl optionally substituted with 1-3 fluoros,
(c) (C1-C6 alkoxy)C1-C6 alkyl- optionally substituted with 1-3 fluoros,
(d) (C1-C6 alkyl)C(═O)—, wherein said alkyl portion is optionally substituted with 1-3 fluoros or with a RgRhN— substituent wherein Rg and Rh are independently H or C1-C6 alkyl,
(e) (hydroxyC2-C6 alkyl)C(═O)— optionally substituted with 1-3 fluoros,
(f) (C1-C6 alkoxy)C(═O)—,
(g) (C3-C6 cycloalkyl)C(═O)—, wherein said cycloalkyl is optionally substituted with one or more substituents independently selected from C1-C6 alkyl, C1-C6 alkoxy, OH, and (C1-C6 alkoxy)C1-C6 alkyl-, or said cycloalkyl is substituted with a 5-6 membered heteroaryl ring having 1-3 ring heteroatoms independently selected from N and O,
(h) Ar1C1-C6 alkyl-,
(i) Ar1(C1-C6 alkyl)C(═O)—, wherein said alkyl portion is optionally substituted with OH, hydroxyC1-C6 alkyl-, C1-C6 alkoxy, RmRnN— or RmRnN—CH2—, wherein each Rm and Rn is independently H or C1-C6 alkyl,
(j) hetAr2C1-C6 alkyl-, wherein said alkyl portion is optionally substituted with 1-3 fluoros,
(k) hetAr2(C1-C6 alkyl)C(═O)— wherein said alkyl portion is optionally substituted with OH, hydroxyC1-C6 alkyl- or C1-C6 alkoxy,
(l) hetAr2C(═O)—,
(m) hetCyc1C(═O)—,
(n) hetCyc1C1-C6 alkyl-,
(o) R3R4NC(═O)—,
(p) Ar1N(R3)C(═O)—,
(q) hetAr2N(R3)C(═O)—,
(r) (C1-C6 alkyl)SO2—, wherein the alkyl portion is optionally substituted with 1-3 fluoros,
(s) Ar1SO2—,
(t) hetAr2SO2—,
(u) N—(C1-C6 alkyl)pyridinonyl,
(v) Ar1C(═O)—;
(w) Ar1O—C(═O)—,
(x) (C3-C6 cycloalkyl)(C1-C6 alkyl)C(═O)—,
(y) (C3-C6 cycloalkyl)(C1-C6 alkyl)SO2—, wherein the alkyl portion is optionally substituted with 1-3 fluoros,
(z) Ar1(C1-C6 alkyl)SO2—,
(aa) hetCyc1-O—C(═O)—,
(bb) hetCyc1CH2C(═O)—,
(cc) hetAr2, or
(dd) C3-C6 cycloalkyl;
Ar1 is phenyl optionally substituted with one or more substituents independently selected from the group consisting of halogen, CN, C1-C6 alkyl (optionally substituted with 1-3 fluoros), C1-C6 alkoxy (optionally substituted with 1-3 fluoros), ReRfN— wherein Re and Rf are independently H, C1-C6 alkyl, (RpRqN)C1-C6 alkoxy- wherein Rp and Rq are independently H or C1-C6 alkyl, and (hetAra)C1-C6 alkyl- wherein hetAra is a 5-6 membered heteroaryl ring having 1-2 ring nitrogen atoms, or Ar1 is a phenyl ring fused to a 5-6 membered heterocyclic ring having 1-2 ring heteroatoms independently selected from N and O;
hetAr2 is a 5-6 membered heteroaryl ring having 1-3 ring heteroatoms independently selected from N, O and S or a 9-10 membered bicyclic heteroaryl ring having 1-3 ring nitrogen atoms, wherein hetAr2 is optionally substituted with one or more substituents independently selected from the group consisting of halogen, CN, C1-C6 alkyl (optionally substituted with 1-3 fluoros), C1-C6 alkoxy (optionally substituted with 1-3 fluoros), (C1-C6 alkoxy)C1-C6 alkyl- (optionally substituted with 1-3 fluoros), ReRfN— wherein Re and Rf are independently H or C1-C6 alkyl, OH, (C1-C6 alkoxy)C1-C6 alkoxy- and C3-C6 cycloalkyl;
hetCyc1 is a 4-6 membered saturated heterocyclic ring having 1-2 ring heteroatoms independently selected from N, O and S wherein said heterocyclic ring is optionally substituted with one or more substituents independently selected from C1-C6 alkoxy and halogen;
R3 is H or C1-C6 alkyl; and
R4 is C1-C6 alkyl,
the process comprising reacting a compound of Formula 12,
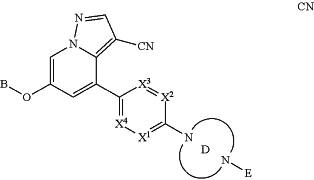 wherein and E is H,
with an aldehyde, in the presence of a reducing agent.
|