| CPC A61K 31/40 (2013.01) [A61P 25/24 (2018.01); C07B 2200/13 (2013.01)] | 44 Claims |
|
1. A method of treating major depressive disorder in a human patient, comprising administering an effective amount of crystalline aticaprant of Form I, II, or III to the human patient, wherein the patient had a previous inadequate response to other antidepressant therapy, wherein:
crystalline Form I is characterized by four or more x-ray diffraction pattern peaks at 2θ (±0.2) of 4.6°, 17.3°, 17.4°, 18.0°, and 24.0°,
crystalline Form II is characterized by four or more x-ray diffraction pattern peaks at 2θ (±0.2) of 3.1°, 19.0°, 24.0°, 24.3°, and 26.2°,
crystalline Form III is characterized by four or more x-ray diffraction pattern peaks at 2θ (±0.2) of 4.1°, 9.0°, 17.6°, 18.0°, and 21.4°,
wherein aticaprant has the following structure:
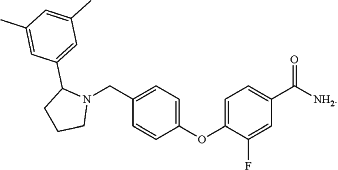 |