| CPC A61K 31/216 (2013.01) [A61P 35/00 (2018.01); C07K 16/32 (2013.01); A61K 2039/505 (2013.01)] | 23 Claims |
|
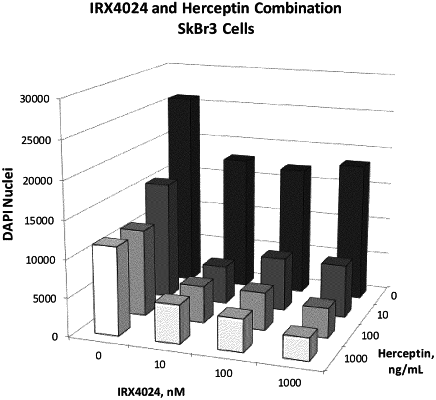
1. A method of treating a patient with Her2+ cancer resistant to at least one Her2-targeted therapeutic agent, the method comprising administering a retinoid X receptor (RXR) agonist of Formula I
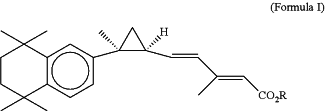 wherein R is H, or lower alkyl of 1 to 6 carbons, or a pharmaceutically-acceptable salt thereof, to the patient;
wherein the Her2+ cancer resistant to a Her2-targeted therapeutic agent is also resistant to the RXR agonist of Formula I; and
the patient has received or is receiving the Her2-targeted therapeutic agent.
|