| CPC A61B 17/0057 (2013.01) [A61B 2017/00004 (2013.01); A61B 2017/00526 (2013.01); A61B 2017/00606 (2013.01); A61B 2017/00623 (2013.01); A61B 2017/00867 (2013.01); A61B 2017/00884 (2013.01); A61B 2017/3425 (2013.01); A61B 2090/3966 (2016.02)] | 19 Claims |
|
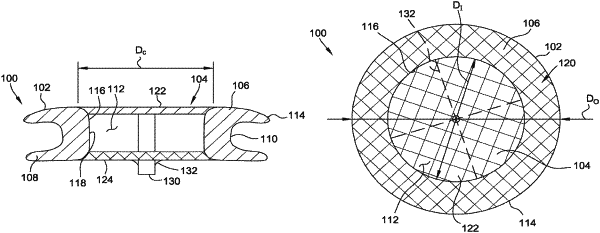
1. An occluder for occluding an atrial septal defect (“ASD”) within an atrial septal wall, the occluder comprising:
a self-expanding mesh frame formed of braided wires of nitinol, the frame including (i) a distal annular flange having a radially outer surface and a radially inner surface, the distal annular flange configured to be positioned with a left atrium and to engage a distal surface of the atrial septal wall, (ii) a proximal annular flange having a radially outer surface and a radially inner surface, the proximal annular flange configured to be positioned within a right atrium and to engage a proximal surface of the atrial septal wall, and (iii) a waist member extending between and connecting the distal annular flange to the proximal annular flange, the waist member configured to engage a surface of the ASD; and
a fabric closure formed of polyethylene terephthalate,
wherein the radially inner surface of the distal annular flange, the waist member, and the radially inner surface of the proximal annular flange define an access passage through the mesh frame, and the fabric closure is connected to the waist member and positioned radially inward of the waist member to (i) restrict bodily fluids from passing through the access passage and to (ii) enable subsequent access through the access passage when the occluder is deployed within the ASD,
wherein the mesh frame includes a plurality of spokes extending radially inwardly from the radially inner surface of the proximal annular flange, the plurality of spokes coupled to an attachment member, the attachment member configured to couple the occluder to a delivery device,
wherein the plurality of spokes define a plurality of access ports leading to the access passage, the plurality of access ports being sized to accommodate medical devices having a diameter of 4 Fr to 36 Fr.
|