| CPC G01N 35/00623 (2013.01) [G01N 35/00693 (2013.01); G01N 35/00732 (2013.01); G01N 35/00871 (2013.01); G01N 35/0092 (2013.01); G16H 10/40 (2018.01); G16H 40/40 (2018.01); G01N 2035/00702 (2013.01); G01N 2035/00752 (2013.01); G01N 2035/00881 (2013.01)] | 13 Claims |
|
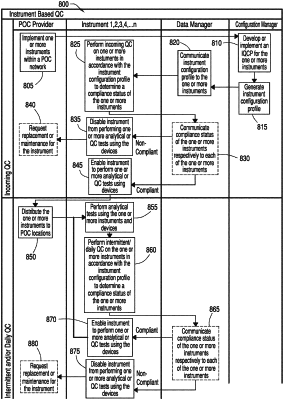
1. A computer implemented method, comprising:
transmitting a request for instrument calibration verification from a data manager to two or more instruments based on a predetermined schedule;
in response to receiving the request for the instrument calibration verification, performing by the two or more instruments the instrument calibration verification according to the request for instrument calibration verification and using: (i) a predetermined number and type of calibration fluids and a predetermined number of sample testing cartridges of a set of sample testing cartridges, or (ii) an electronic simulator, to generate calibration verification data;
transmitting the calibration verification data from the two or more instruments to the data manager;
based on whether the calibration verification data is within range of predetermined calibration target values, determining at the data manager a compliance status for each of the two or more instruments as either compliant or non-compliant;
storing at the data manager the determined compliance status for each of the two or more instruments in a data table, the data table comprising: (i) identification information associated with instruments of the two or more instruments, and (ii) the compliance status for each of the two or more instruments;
transmitting the data table from the data manager to the two or more instruments; and
enabling use of a first instrument from the two or more instruments for performing one or more analytical tests on a first biological sample when the compliance status for the first instrument stored in the data table indicates the first instrument from the two or more instruments is in compliance; or
at least partially disabling use of a second instrument from the two or more instruments for performing the one or more analytical tests on a second biological sample when the compliance status for the second instrument stored in the data table indicates the second instrument from the two or more instruments is not in compliance.
|