| CPC G01N 30/26 (2013.01) [B01D 15/1878 (2013.01); B01D 15/22 (2013.01); B01D 15/361 (2013.01); B01D 15/3847 (2013.01); G01N 2030/027 (2013.01)] | 5 Claims |
|
1. A method of separating analytes in a sample, the method comprising:
introducing a sample comprising the analytes into a chromatographic system comprising:
metallic flow path components disposed in an interior of the chromatographic system defining a flow path, at least a portion of the metallic flow path components having an active coating; and
a chromatographic column having a stationary phase material in an interior of the chromatographic column that facilitates separation of the analytes in the sample through interaction with at least one analyte in the sample, wherein the stationary phase material is distinct from the metallic flow path components;
wherein the metallic flow path components having the active coating are not inert to the analytes; and
wherein the active coating comprises an alkylsilyl coating of Formula I:
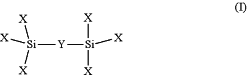 wherein each X is independently selected from (C1-C6)alkoxy, —NH(C1-C6)alkyl, —N((C1-C6)alkyl)2, OH, ORA, RB, RC, RD and halo;
RA represents a point of attachment to the interior surfaces of the fluidic system and at least one X is ORA;
RB is absent or represents a hydrophobicity modifier;
RC represents a charge modifier and at least one X is RC;
RD is absent, a chelator, or a crown ether; and
Y is a bridging moiety selected from (C1-C20)alkyl, —O[(CH2)2O]1-20—, —(C1-C10)[NH(CO)NH(C1-C10)]1-20—, or —(C1-C10)[alkylphenyl(C1-C10)alkyl]1-20—.
|