| CPC C07H 15/26 (2013.01) [C07H 21/00 (2013.01)] | 31 Claims |
|
1. A compound of Formula (II):
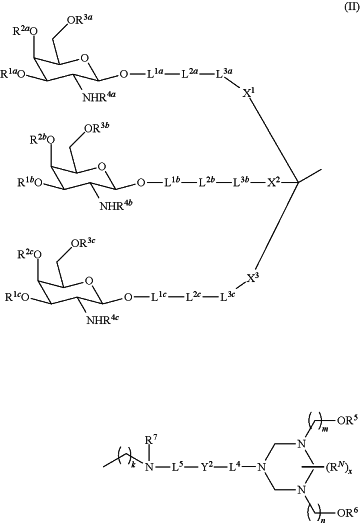 or a pharmaceutically acceptable salt thereof, wherein
each of R1a, R1b, R1c, R2a, R2b, R2c, R3a, R3b, and R3c is independently hydrogen, benzyl, or —C(═O)R8;
each of R4a, R4b and R4c is independently —C(═O)C1-6 alkyl or —C(═O)C1-6 haloalkyl;
R5 is a hydroxy protecting group;
R6 is hydrogen, a phosphoramidite moiety, —C(═O)CH2CH2C(═O)R6A, or —P(OR6B)NR6CR6D;
Y2 is a bond, NR7, NR7C(═O), C(═O)O, or O;
each of X1, X2 and X3 is independently —O(CH2)1-3—, —NH(CH2)1-3—, —C(═O)(CH2)1-3— or —NHC(═O)(CH2)1-3-;
each of L1a, L1b, L1c, L2a, L2b, L2c, L3a, L3b, and L3c is independently a bond, —C(═O)—, —C(═S)—, —S(═O)2—, —C(═O)NR9—, —C(═S)NR9—, —C(═O)O—, —C(═S)O—, —NR9C(═O)NR9—, —NR9C(═S)NR9—, —OP(═O)(OH)O—, —OP(═S)(OH)O—, —O—, —S—, —NR9—, optionally substituted C1-10 alkylene, optionally substituted C6-10 arylene, optionally substituted C3-10 cycloalkylene, optionally substituted 5-10 membered hetetroarylene, optionally substituted 5 to 10 membered heterocyclylene, or optionally substituted 2 to 15 membered heteroalkylene wherein one or more carbon atoms are replaced with C(═O), O, S or N, provided that at least one of L1a, L1b and L1c is not a bond, at least one of L2a, L2b and L2c is not a bond, and at least one of L3a, L3b and L3c is not a bond;
L4 is a bond, optionally substituted C1-8 alkylene or —(CH2)1-3(OCH2CH2)1-5OCH2-;
L5 is a bond, optionally substituted C1-10 alkylene, or optionally substituted 2 to 15 membered heteroalkylene wherein one or more carbon atoms are replaced with C(═O), O, S or N;
each of R7 and R9 is independently hydrogen, unsubstituted C1-6 alkyl, or substituted C1-6 alkyl;
each R8 is independently C1-6 alkyl, C1-6 haloalkyl, or optionally substituted phenyl;
R6A is —OH, —OR10 or —NR11R12;
each of R6B, R6C and R6D is independently H, C1-6 haloalkyl, or unsubstituted or substituted C1-6 alkyl;
R10 is unsubstituted or substituted C1-6 alkyl, or a hydroxy protecting group;
each of R11 and R12 is independently H, unsubstituted or substituted C1-6 alkyl, or an amino protecting group;
each of m and n is independently 1, 2, 3, 4, 5 or 6;
k is 0 or 1;
x is 0, 1, 2, 3, 4, 5 or 6; and
each RN is independently halo, cyano, hydroxy, amino, unsubstituted C1-6 alkyl, substituted C1-C6 alkyl, C1-C6 haloalkyl, hydroxy(C1-C6 alkyl), amino(C1-C6 alkyl), (C1-C6 alkyl)amino, C1-C6 alkoxy, C1-C6 haloalkoxy, (C1-C6 alkoxy)C1-C6 alkyl, or —O(C1-C6 alkoxy)C1-C6 alkyl, or two adjacent RN attached to the same carbon atom form oxo (═O).
|