| CPC C07D 417/12 (2013.01) [C07D 401/12 (2013.01); C07D 401/14 (2013.01); C07D 403/12 (2013.01); C07D 405/14 (2013.01); C07D 413/12 (2013.01); C07D 413/14 (2013.01); C07D 417/14 (2013.01); C07D 471/04 (2013.01); C07D 487/04 (2013.01); C07D 513/04 (2013.01)] | 10 Claims |
|
1. A compound of formula (I):
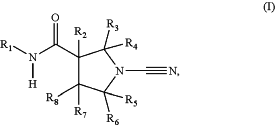 a tautomer thereof, or a pharmaceutically acceptable salt of said compound or tautomer, wherein:
R1 represents a 9-membered bicyclic heteroaryl ring comprising 1 to 3 heteroatoms independently selected from nitrogen, oxygen and sulphur, and which is substituted with 1 to 4 Q1-(R9)n;
R2, R3, R4, R5, R6 and R8 are each independently selected from hydrogen, C1-C3 alkyl and C1-C3 alkoxy, wherein said alkyl and alkoxy are optionally substituted with halo, C1-C3 alkyl or C1-C3 alkoxy;
R7 is selected from hydrogen, methyl, CF3, phenyl, pyridyl and pyrimidyl;
n is 0 or 1;
when n is 0, Q1 is selected from hydrogen, halogen, cyano, oxo, nitro, C1-C6 alkoxy, C1-C6 haloalkoxy, C1-C6 hydroxyalkyl, —SO2R11, —NR11R12, —NR11COR12, —NR10CONR11R12, —CO2R11, —NR11CO21R12, —SO2NR11R12, —C(O)R11, —NR11SO2R12, NR11SO2NR13R14, SO2NR11, —C1-C6 alkyl and C1-C2 haloalkyl;
when n is 1, Q1 is selected from a covalent bond, —CONR10—, —NR10CO—, an oxygen atom, —S(O)m—, —CO—, C1-C6 alkylene and —C2-C6 alkenylene;
m is 0, 1 or 2;
R9 represents a 3 to 10-membered heterocyclyl, heteroaryl, aryl or cycloalkyl ring;
R10, R11 and R12 are each independently selected from hydrogen, C1-C6 alkyl and C1-C6 alkylene;
wherein R9 is optionally substituted with one or more substituents selected from halogen, C1-C6 haloalkyl, C1-C6 alkoxy, C1-C6 haloalkoxy, C1-C6 alkyl, C2-C6 alkenyl, C2-C6 alkynyl, C1-C6 hydroxyalkyl, oxo, cyano, nitro, -Q2-NR13CONR14R15, -Q2-NR13R14, -Q2-NR13COR14, -Q2-COR13, -Q2-SO2R13, -Q2-CONR13, Q2-CONR13R14, -Q2-CO2R13, -Q2-SO2NR13R14, -Q2-NR13SO2R14 and -Q2-NR13SO2NR14R15; wherein said alkyl and alkoxy are optionally substituted with halo, deutero, C1-3 alkyl, hydroxy, C1-3 alkoxy, cyano, amino, nitro, oxo, C1-3 alkylamino, C2-6 alkenylamino, di-C1-3 alkylamino, C1-3 acylamino, di-C1-3 acylamino, carboxy, C1-3 alkoxycarbonyl, carbamoyl, mono-C1-3 carbamoyl or di-C1-3 carbamoyl, or any of the above in which a hydrocarbyl moiety is itself substituted by halo;
or R9 is optionally substituted with a 3 to 10-membered heterocyclyl, heteroaryl, aryl or cycloalkyl ring, either directly attached or via linking group selected from oxygen, carbonyl, C1-C6 alkylene and C1-C6 alkyleneoxy;
Q2 represents a covalent bond, C1-C6 alkylene or C2-C6 alkenylene; and
R13, R14 and R15 are each independently selected from hydrogen, C1-C6 alkyl and C3-C6 cycloalkyl.
|