| CPC C07D 401/14 (2013.01) [A61P 13/12 (2018.01); C07D 401/06 (2013.01); C07D 403/06 (2013.01); C07D 403/14 (2013.01); C07D 405/14 (2013.01); C07D 417/14 (2013.01); C07D 471/04 (2013.01); C07D 487/04 (2013.01); C07D 495/04 (2013.01)] | 20 Claims |
|
1. A method of treating cell death in a subject after surgery, comprising administering to the subject an effective amount of a compound, or a pharmaceutically acceptable salt thereof, or a pharmaceutical composition comprising the compound, or a pharmaceutically acceptable salt thereof, and a pharmaceutically acceptable carrier; and wherein said compound is represented by the following structural formula:
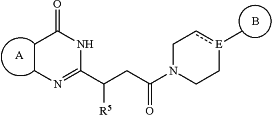 or a pharmaceutically acceptable salt thereof, wherein:
Ring A is phenyl or 5-6 membered heteroaryl; each of which is optionally substituted with one or two substituents selected from the group consisting of -halogen, —CN, —NO2, —NRaRb, —S(O)iRa, —NRaS(O)iRb, —S(O)iNRaRb, —C(═O)ORa, —OC(═O)ORa, —C(═S)ORa, —O(C═S)Ra, —C(═O)NRaRb, —NRaC(═O)Rb, —C(═S)NRaRb, —NRaC(═S)Rb, —NRa(C═O)ORb, —O(C═O)NRaRb, —NRa(C═S)ORb, —O(C═S)NRaRb, —NRa(C═O)NRaRb, —NRa(C═S)NRaRb, —C(═S)Ra, —C(═O)Rb, halo(C1-C5)alkyl and (C1-C5)alkyl, wherein the (C1-C5)alkyl is optionally substituted one or two groups selected from —CN, —NO2, —NRaRb, —S(O)iRa, —NRaS(O)iRb, —S(O)iNRaRb, —C(═O)ORa, —OC(═O)ORa, —C(═S)ORa, —O(C═S)Ra, —C(═O)NRaRb, —NRaC(═O)Rb, —C(═S)NRaRb, —NRaC(═S)Rb, —NRa(C═O)ORb, —O(C═O)NRaRb, —NRa(C═S)ORb, —O(C═S)NRaRb, —NRa(C═O)NRaRb, —NRa(C═S)NRaRb, —C(═S)Ra, and —C(═O)Ra;
each Ra and each Rb are independently selected from —H and (C1-C5)alkyl optionally substituted with hydroxyl or (C1-C3)alkoxy;
Rc is —H, halo(C1-C5)alkyl or (C1-C5)alkyl, wherein the (C1-C5)alkyl is optionally substituted with hydroxyl or (C1-C3)alkoxy;
i is 0, 1, or 2;
Ring B is phenyl, 5-6 membered heteroaryl or 5-6 membered heterocyclyl, each optionally substituted with one or two substituents represented by R3;
 is absent or a bond; is absent or a bond;E is N or CH when
 is absent or E is C when is absent or E is C when  is a bond; is a bond; is optionally substituted with (C1-C5)alkyl or hydroxy (C1-C5)alkyl;
each R3 is independently selected from the group consisting of -halogen, —CN, —NO2, —ORd, —S(O)iRe, —C(═NRe)NReRf, —NReS(O)iRf, —S(O)iNReRf, —C(═O)ORe, —OC(═O)ORe, —C(═S)ORe, —O(C═S)Re, —C(═O)NReRf, —NReC(═O)Rf, —C(═S)NReRf, —NReC(═S)Rf, —NRe(C═O)ORf, —O(C═O)NReRf, —NRe(C═S)ORf, —O(C═S)NReRf, —NRe(C═O)NReRf, —NRe(C═S)NReRf, —C(═S)Re, —C(═O)Re, halo(C1-C5)alkyl, and (C1-C5)alkyl, wherein the (C1-C5)alkyl represented by R3 is optionally substituted with —CN, —NO2, —ORe, —NReRf, —S(O)iRe, —NReS(O)iRf, —S(O)iNReRf, —C(═O)ORe, —OC(═O)ORe, —C(═S)ORe, —O(C═S)Re, —C(═O)NReRf, —NReC(═O)Rf, —C(═S)NReRf, —NReC(═S)Rf, —NRe(C═O)ORf, —O(C═O)NReRf, —NRe(C═S)ORf, —O(C═S)NReRf, —NRe(C═O)NReRf, —NRe(C═S)NReRf, —C(═S)Re, or —C(═O)Re;
Rd is —H, halo(C1-C5)alkyl or (C1-C5)alkyl, wherein the (C1-C5)alkyl is optionally substituted with hydroxyl or (C1-C3)alkoxy;
each Re is independently selected from the group consisting of —H and (C1-C5)alkyl optionally substituted with hydroxyl or (C1-C3)alkoxy;
each Rf is independently selected from the group consisting of —H, (C1-C5)alkyl optionally substituted with hydroxyl or (C1-C3)alkoxy, (C3-C6)cycloalkyl optionally substituted with (C1-C2) alkyl, and 4-6 membered oxygen-containing heterocyclyl optionally substituted with (C1-C2) alkyl; or
—NReRf taken together is a 4-6 membered heterocyclyl optionally substituted with (C1-C2) alkyl; or
—C(═NRe)NReRf taken together is a 4-6 membered heterocyclyl optionally substituted with Re;
R5 is —H or (C1-C5)alkyl; and
i is 0, 1, or 2,
wherein 5-6 membered heteroaryl refers to a monocyclic aromatic ring group having five or six ring atoms selected from carbon and 1 to 4 heteroatoms selected from oxygen, nitrogen and sulfur; and
5-6 membered heterocyclyl refers to a monocyclic non-aromatic ring radical containing 5-6 ring atoms selected from carbon atom and 1 or 2 heteroatoms, each heteroatom is independently selected from nitrogen, quaternary nitrogen, oxidized nitrogen (NO), oxygen, sulfur, sulfoxide and sulfone.
|