| CPC C07D 231/54 (2013.01) [C07D 235/02 (2013.01); C07D 401/04 (2013.01); C07D 401/06 (2013.01); C07D 401/10 (2013.01); C07D 401/14 (2013.01); C07D 403/04 (2013.01); C07D 403/10 (2013.01); C07D 403/14 (2013.01); C07D 405/04 (2013.01); C07D 409/04 (2013.01); C07D 413/10 (2013.01); C07D 413/14 (2013.01); C07D 417/04 (2013.01); C07D 417/14 (2013.01)] | 37 Claims |
|
1. A compound of the formula:
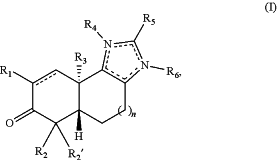 wherein:
n is 0, 1, or 2;
R1 is cyano, fluoro, —CF3, or —C(O)Ra, wherein:
Ra is hydroxy or amino; or
alkoxy(C≤6), alkylamino(C≤6), dialkylamino(C≤6), or a substituted version of any of these groups;
R2 is hydrogen; or
alkyl(C≤12), cycloalkyl(C≤12), alkenyl(C≤12), alkynyl(C≤12), aryl(C≤18), aralkyl(C≤18), heteroaryl(C≤18), heteroaralkyl(C≤18), or a substituted version of any of these groups;
R2′ is hydrogen;
R3 is alkyl(C≤12), alkenyl(C≤12), aryl(C≤12), aralkyl(C≤12), or a substituted version of any of these groups;
R4 and R5 are each independently absent; or
cycloalkyl(C≤12), heterocycloalkyl(C≤12), aryl(C≤12), aralkyl(C≤12), heteroaryl(C≤12), heteroaralkyl(C≤12), -arenediyl(C≤12)-alkyl(C≤12), -arenediyl(C≤12)-aryl(C≤12), -arenediyl(C≤12)-heteroaryl(C≤12), -arenediyl(C≤12)-heterocycloalkyl(C≤12), -arenediyl(C≤12)-cyclo-alkyl(C≤12), -heteroarenediyl(C≤12)-alkyl(C≤12), -heteroarenediyl(C≤12)-aryl(C≤12), -hetero-arenediyl(C≤12)-heteroaryl(C≤12), -heteroarenediyl(C≤12)-hetero-cycloalkyl(C≤12), -heteroarenediyl(C≤12)-cycloalkyl(C≤12), -heterocycloalkanediyl(C≤12)-aryl(C≤12), -heterocycloalkanediyl(C≤12)-heteroaryl(C≤12), or a substituted version of any of these groups; or
a group of the formula:
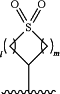 wherein 1 and m are each 0, 1, 2, or 3;
R6 is absent or amino; or
alkylamino(C≤12), dialkylamino(C≤12), cycloalkylamino(C≤12), dicycloalkylamino(C≤12), alkyl(cycloalkyl)amino(C≤12), arylamino(C≤12), diarylamino(C≤12), alkyl(C≤12), cycloalkyl(C≤12), -alkanediyl(C≤12)-cycloalkyl(C≤12), -alkanediyl(C≤18)-aralkoxy(C≤18), heterocycloalkyl(C≤12), aryl(C≤18), -arenediyl(C≤12)-alkyl(C≤12), aralkyl(C≤18), -arenediyl(C≤18)-heterocycloalkyl(C≤12), heteroaryl(C≤18), -heteroarenediyl(C≤12)-alkyl(C≤12), heteroaralkyl(C≤18), acyl(C≤12), alkoxy(C≤12), or a substituted version of any of these groups;
or a pharmaceutically acceptable salt thereof.
|