| CPC A61N 1/3937 (2013.01) [A61B 5/318 (2021.01); A61B 5/48 (2013.01); A61B 5/1113 (2013.01); A61B 5/1118 (2013.01)] | 20 Claims |
|
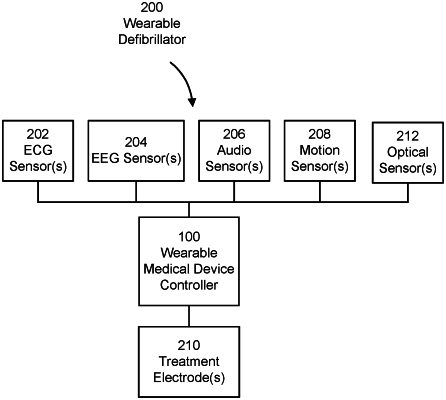
1. An external wearable defibrillator for monitoring a patient for cardiac-related events comprising:
a plurality of ECG sensors configured to be disposed about a torso of the patient;
a plurality of therapy electrodes configured to be disposed on a first location and a second location on the patient;
at least one motion sensor;
wherein the plurality of ECG sensors, the plurality of therapy electrodes, and the at least one motion sensor are configured to be worn continuously by the patient for an extended period of time;
a wearable medical device controller comprising at least one processor configured to
receive ECG data from the plurality of ECG sensors;
receive motion data from the at least one motion sensor,
using the ECG data received from the plurality of ECG sensors, identify at least one of ventricular tachycardia or ventricular fibrillation,
initiate a treatment protocol comprising a defibrillation shock after identifying at least one of ventricular tachycardia or ventricular fibrillation,
using at least one of the ECG data received from the plurality of ECG sensors or the motion data received from the at least one motion sensor, identify a predetermined pattern for a precursor of syncope, wherein the predetermined pattern for the precursor of syncope comprises bradycardia,
record the predetermined pattern for the precursor of syncope in a memory, and
perform an intervening action to address the predetermined pattern for the precursor of syncope.
|