|
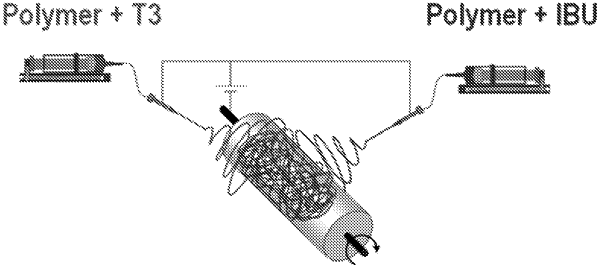
1. Electrospun polymeric fibers in the form of scaffold, wherein part or all of said fibers are loaded with the promyelinating agent 3,3,5-Triiodo-L-thyronine (T3) and another part or all of said fibers are loaded with an anti-inflammatory agent, for a local release of said promyelinating agent and said anti-inflammatory agent in a method of treating a spinal cord injury, wherein said electrospun polymeric fibers are suitable to be implanted directly on top of a lesion, wherein the local release of said promyelinating agent and said anti-inflammatory agent in a method of treating a spinal cord injury occurs over an estimated time of 8 to 14 days.
|