| CPC A61K 9/0065 (2013.01) [A61K 9/146 (2013.01); A61K 9/20 (2013.01); A61K 47/34 (2013.01); A61K 47/46 (2013.01); A61M 31/002 (2013.01); C08G 18/12 (2013.01); C08G 18/4202 (2013.01); C08G 18/4277 (2013.01); C08G 18/73 (2013.01)] | 20 Claims |
|
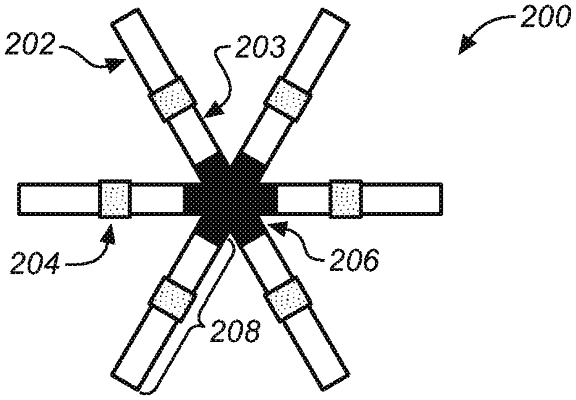
1. A gastric residence system for administration to a stomach of a patient, comprising:
a central elastomer component, wherein the central elastomer component is mono-concave, bi-concave, concavo-convex, or toroidal;
a plurality of at least three carrier polymer-agent components comprising a carrier polymer and a therapeutic agent or a salt thereof,
wherein each of the plurality of carrier polymer-agent components comprises an elongate member comprising a proximal end, a distal end, and an outer surface therebetween;
wherein the proximal end of each elongate member is attached to the central elastomer component and projects radially from the central elastomer component, each elongate member having the distal end not attached to the elastomer component and located at a larger radial distance from the central elastomer component than the proximal end;
wherein the central elastomer component is attached directly or indirectly to each elongate member by an intercomponent anchor, wherein the intercomponent anchor is a separate component from the central elastomer component and each of the elongate members;
wherein a first portion of each intercomponent anchor is located within the central elastomer component, and a second portion of each intercomponent anchor is located within:
a) a corresponding first segment of interfacing polymer, wherein each corresponding first segment of interfacing polymer is also attached directly or indirectly to a corresponding one of the elongate members;
b) a corresponding segment of linker, wherein each corresponding segment of linker is also attached directly or indirectly to a corresponding one of the elongate members; or
c) a corresponding one of the elongate members;
wherein
i) the central elastomer component is overmolded over the first portions of the intercomponent anchors, or
ii) the first segment of each interfacing polymer, a first segment of the corresponding segment of each linker, or a first segment of each elongate member is overmolded over the corresponding second portion of the intercomponent anchors;
wherein the gastric residence system has a compacted form when within a container that provides a constraining force, and is suitable for administration orally or through a feeding tube in the compacted form; and has an uncompacted form resulting from elastic recoil of the gastric residence system when released from the constraining force provided by the container in the stomach of the patient;
wherein the gastric residence system is configured to be retained in the stomach for a period of at least about 24 hours; and
wherein the gastric residence system is configured to release a therapeutically effective amount of the therapeutic agent or the salt thereof over at least a portion of the period in which the gastric residence system is retained in the stomach.
|