| CPC A61K 31/551 (2013.01) [C07D 495/14 (2013.01); C07D 519/00 (2013.01)] | 13 Claims |
|
1. A compound of formula (I)
 wherein
R1 is selected from the group consisting of C1-4-alkyl and C3-4-cycloalkyl,
wherein the C1-4-alkyl group of R1 is optionally substituted with 1 to 3 F;
n is selected from the group consisting of 0, 1, 2, and 3;
R2 is independently selected from the group consisting of F, Cl, Br, I, C1-4-alkyl, C3-4-cycloalkyl, —CN, —CONH2, —CONH(C1-4-alkyl), —CON(C1-4-alkyl)2, —COOH, —COO—C1-4-alkyl, NH2, OH, —O—C1-4-alkyl, and —S(O)r-C1-4-alkyl with r=0, 1, or 2;
wherein the C1-4-alkyl group of R2 is optionally substituted with 1 to 3 F or with 1 —CN, with 1 OH, or with 1 O—C1-4-alkyl, and
wherein the —O—C1-4-alkyl group of R2 is optionally substituted with 1 to 3 F;
R3 is selected from the group consisting of H and C1-4-alkyl optionally substituted with 1 to 5 F; and
R4 is selected from the group consisting of C1-6-alkyl
optionally substituted with 1 to 3 F or optionally substituted with 1 to 2 substituents independently selected from
—CN, —CONH2, —CONH(C1-4-alkyl), —CON(C1-4-alkyl)2, —COOH, —COO—C1-4-alkyl, C1-3-alkyl-CO—NH—, C1-3-alkyl-S(═O)2—NH—, OH, and —O—C1-3-alkyl optionally substituted with 1 to 3 F;
or
R4 is selected from the group consisting of —C0-3-alkylene-C3-10-cycloalkyl and —C0-3-alkylene-C3-10-heterocyclyl,
wherein said alkylene moiety of the —C0-3-alkylene-C3-10-cycloalkyl and —C0-3-alkylene-C3-10-heterocyclyl groups of R4 is optionally substituted with 1 to 2 substituents selected from F and CH3,
wherein 1 CH2 group of said alkylene moiety of the —C0-3-alkylene-C3-10-cycloalkyl and —C0-3-alkylene-C3-10-heterocyclyl groups of R4 is optionally replaced by a
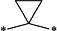 moiety,
wherein said cycloalkyl moiety of the —C0-3-alkylene-C3-10-cycloalkyl group of R4 and said heterocyclyl moiety of the —C0-3-alkylene-C3-10-heterocyclyl group of R4 are saturated mono- or bicyclic ring systems,
wherein said heterocyclyl moiety of the —C0-3-alkylene-C3-10-heterocyclyl group of R4 contains 1 to 2 ring members independently selected from N, NH, N(C1-4-alkyl), NCO(C1-4-alkyl), NCOO(C1-4-alkyl), NS(═O)2(C1-4-alkyl), N-phenyl, N-pyridinyl, N-pyrimidinyl, and O, and optionally 1 ring member selected from C═O, and S(═O)r, with r=0, 1, or 2,
provided that said heterocyclyl moiety of the —C0-3-alkylene-C3-10-heterocyclyl group of R4 does not contain any heteroatom-heteroatom bonds other than N—N, N—O, and N—S(═O)r=1,2 between ring members, and
wherein said cycloalkyl moiety of the —C0-3-alkylene-C3-10-cycloalkyl group of R4 and said heterocyclyl moiety of the —C0-3-alkylene-C3-10-heterocyclyl group of R4 are optionally substituted with 1 to 2 F and optionally substituted with 1 to 2 substituents independently selected from
a) Cl,
b) —CN,
c) —CONH2,
d) —CONH(C1-4-alkyl),
e) —CON(C1-4-alkyl)2,
f) —COOH,
g) —COO—C1-4-alkyl,
h) OH,
i) —O—C1-3-alkyl optionally substituted with 1 to 3 F,
j) S(═O)2—C1-4-alkyl and
k) C1-4-alkyl optionally substituted with 1 to 3 F or with 1 substituent selected from —CN, OH, —O—C1-4-alkyl;
or
R4 is selected from the group consisting of —C0-3-alkylene-phenyl and —C0-3-alkylene-heteroaryl,
wherein said alkylene moiety of the —C0-3-alkylene-phenyl and —C0-3-alkylene-heteroaryl groups of R4 is optionally substituted with 1 to 2 substituents selected from F and CH3,
wherein 1 CH2 group of said alkylene moiety of the —C0-3-alkylene-phenyl and —C0-3-alkylene-heteroaryl groups of R4 is optionally replaced by a
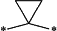 moiety or by a
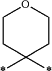 moiety, or
wherein 1 —CH2—CH2— group of said alkylene moiety of the —C0-3-alkylene-phenyl and —C0-3-alkylene-heteroaryl groups of R4 is optionally replaced by a
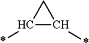 moiety or by a
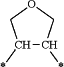 moiety,
wherein said heteroaryl moiety of the —C0-3-alkylene-heteroaryl group of R4 is a 5-membered monocycle containing 1 ring member selected from N, NH, O, and S and optionally further containing 1 to 2 ring members N, or a 6-membered monocycle containing 1 to 2 ring members N, and
wherein said phenyl moiety of the —C0-3-alkylene-phenyl group of R4 and said heteroaryl moiety of the —C0-3-alkylene-heteroaryl group of R4 are optionally substituted with 1 to 3 substituents independently selected from
a) F, Cl, Br,
b) C3-4-cycloalkyl,
c) —CN,
d) —CONH2,
e) —CONH(C1-4-alkyl),
f) —CON(C1-4-alkyl)2,
g) —COOH,
h) —COO—C1-4-alkyl,
i) —NHCO—C1-4-alkyl,
j) —NHS(═O)2—C1-4-alkyl,
k) —S(═O)r-C1-4-alkyl with r=0, 1, or 2,
l) —O—C1-4-alkyl optionally substituted with 1 to 3 F, and
m) C1-4-alkyl optionally substituted with 1 to 3 F or with 1 substituent selected from —CN, OH, and —O—C1-4-alkyl,
or
R4 is selected from the group consisting of 7- to 12-membered fused bicyclic aryl, heteroaryl, or heterocyclyl
wherein said bicyclic aryl, heteroaryl, or heterocyclyl of R4 consists of one non-aromatic ring that is attached to the amide N atom in formula (I) and optionally contains 1 to 2 ring members independently selected from N—, NH, N(C1-4-alkyl), N(CO—C1-3-alkyl), N(S(═O)2-C1-3-alkyl), and O, and
optionally contains 1 ring member selected from C═O and S(═O)r, with r=0, 1, or 2, and of
one aromatic ring selected from phenyl, pyrrole, furan, and thiophene in each of which 1 to 2 CH ring members are optionally replaced with N,
wherein said bicyclic aryl, heteroaryl, or heterocyclyl of R4 is optionally substituted with 1 to 4 F,
is optionally substituted with 1 to 4 C1-3-alkyl optionally substituted with 1 to 4 F, and
is optionally substituted with 1 to 2 substituents selected from the group consisting of Cl, —CN, —CONH2, —CONH(C1-4-alkyl), —CON(C1-4-alkyl)2, —COOH, —COO—C1-4-alkyl, HO—C1-3-alkylene-, C1-3-alkyl-O—C1-3-alkylene-, NH2, C1-3-alkyl-CO—NH—, C1-3-alkyl-S(═O)2-NH—, OH, and C1-3-alkyl-O— optionally substituted with 1 to 3 F;
or
R3 and R4 are selected from the group in which R3 and R4, together with the amide N atom they are attached to, form a saturated 3- to 8-membered monocyclic heterocyclyl optionally further containing 1 to 2 ring members independently selected from NH, N(C1-4-alkyl), N(CO—C1-3-alkyl), N(S(═O)2-C1-3-alkyl), and O, and
optionally containing 1 ring member selected from C═O and S(═O)r, with r=0, 1, or 2, provided that said saturated 3- to 8-membered monocyclic heterocyclyl formed by R3 and R4 together with the amide N atom they are attached to does not contain any heteroatom-heteroatom bonds other than N—N, N—O, and N—S(═O)r=1,2 between ring members,
wherein said heterocyclyl formed by R3 and R4 together with the amide N atom they are attached to is optionally substituted with 1 to 4 F,
is optionally substituted with 1 to 4 C1-3-alkyl optionally substituted with 1 to 3 F, and
is optionally substituted with 1 to 2 substituents selected from Cl, —CN, —CONH2, —CONH(C1-4-alkyl), —CON(C1-4-alkyl)2, —COOH, —COO—C1-4-alkyl, HO—C1-3-alkylene-, C1-3-alkyl-O—C1-3-alkylene-, C1-3-alkyl-CO—NH—, C1-3-alkyl-S(═O)2—NH—, OH, and C1-3-alkyl-O— optionally substituted with 1 to 3 F;
or
R3 and R4 are selected from the group in which R3 and R4, together with the amide N atom they are attached to, form a saturated 5- to 12-membered bicyclic heterocyclyl optionally further containing 1 to 3 ring members independently selected from N—, NH, N(C1-4-alkyl), N(CO—C1-3-alkyl), N(S(═O)2—C1-3-alkyl), and O, and
optionally containing 1 ring member selected from C═O and S(═O)r, with r=0, 1, or 2, provided that said saturated 5- to 12-membered bicyclic heterocyclyl formed by R3 and R4 together with the amide N atom they are attached to does not contain any heteroatom-heteroatom bonds other than N—N, N—O, and N—S(═O)r=1,2 between ring members
wherein said saturated 5- to 12-membered bicyclic heterocyclyl formed by R3 and R4 together with the amide N atom they are attached to is optionally substituted with 1 to 6 F,
is optionally substituted with 1 to 4 C1-3-alkyl optionally substituted with 1 to 3 F, or is optionally substituted with 1 to 2 substituents selected from Cl, —CN, —CONH2, —CONH(C1-4-alkyl), —CON(C1-4-alkyl)2, —COOH, —COO—C1-4-alkyl, HO—C1-3-alkylene-, C1-3-alkyl-O—C1-3-alkylene-, C1-3-alkyl-CO—NH—, C1-3-alkyl-S(═O)2-NH—, OH, and C1-3-alkyl-O—;
or a salt thereof.
|