| CPC A61K 31/4155 (2013.01) [A61K 9/1611 (2013.01); A61K 9/1617 (2013.01); A61K 9/1635 (2013.01); A61K 9/1694 (2013.01); A61K 9/2009 (2013.01); A61K 9/2013 (2013.01); A61K 9/2027 (2013.01); A61K 9/2054 (2013.01); A61K 9/2086 (2013.01); A61K 9/2095 (2013.01); A61K 9/2813 (2013.01); A61K 9/284 (2013.01); A61K 9/2866 (2013.01); A61K 31/155 (2013.01); A61K 31/40 (2013.01); A61K 31/403 (2013.01); A61K 31/4035 (2013.01); A61K 31/4439 (2013.01); A61K 31/4985 (2013.01); A61K 31/522 (2013.01); A61K 31/7034 (2013.01); A61K 45/06 (2013.01); A61K 47/38 (2013.01); A61P 3/10 (2018.01); C07D 231/38 (2013.01); C07D 403/12 (2013.01)] | 63 Claims |
|
1. A fixed dose combination formulation, comprising:
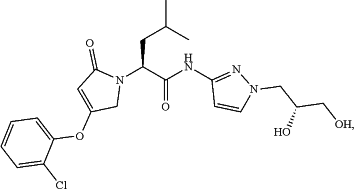
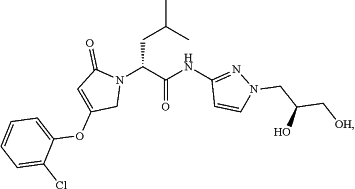
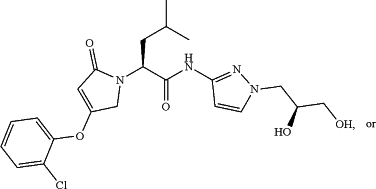
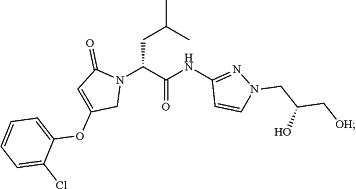
(a) a glucokinase activator, which is a compound represented by the following formulae, or a pharmaceutically acceptable salt, an isotope labeled analogue, a crystalline form, a hydrate, a solvate, or a diastereomeric or enantiomeric form thereof,
| |||||||||||
(b) a SGLT-2 inhibitor; and
(c) one or more excipients.
|