| CPC A61B 18/082 (2013.01) [A61B 18/04 (2013.01); A61B 18/10 (2013.01); A61B 90/04 (2016.02); A61B 2018/00023 (2013.01); A61B 2018/00029 (2013.01); A61B 2018/00047 (2013.01); A61B 2018/00083 (2013.01); A61B 2018/00101 (2013.01); A61B 2018/00285 (2013.01); A61B 2018/00547 (2013.01); A61B 2018/00577 (2013.01); A61B 2018/00821 (2013.01); A61B 2018/00869 (2013.01); A61B 2018/00875 (2013.01); A61B 2018/00898 (2013.01); A61B 2018/00982 (2013.01); A61B 2018/048 (2013.01); A61B 2090/0427 (2016.02); A61B 2090/0436 (2016.02)] | 17 Claims |
|
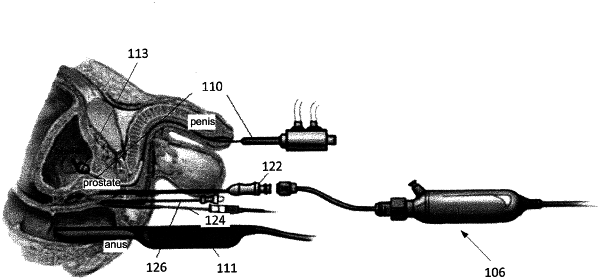
1. A medical device comprising:
a needle;
a first electrode disposed at a distal tip of the needle, wherein the first electrode is configured to measure a first electrical impedance of a first tissue contacting the first electrode, and wherein the distal tip of the needle is the first electrode;
a second electrode disposed on the needle, proximally of the first electrode, wherein the second electrode is configured to measure a second electrical impedance of a second tissue contacting the second electrode; and
an electrically insulative material disposed between the first electrode and the second electrode; and
an electronic controller configured to use the first electrical impedance and the second electrical impedance in order to determine whether the first electrode and the second electrode are disposed within a prostate.
|