| CPC H10K 85/6572 (2023.02) [H10K 85/324 (2023.02); H10K 85/624 (2023.02); H10K 85/633 (2023.02); H10K 85/653 (2023.02); H10K 85/654 (2023.02); H10K 85/6574 (2023.02); H10K 85/6576 (2023.02); H10K 50/11 (2023.02); H10K 2101/10 (2023.02); H10K 2101/30 (2023.02); H10K 2101/40 (2023.02)] | 20 Claims |
|
1. An organic light-emitting device comprising:
a first electrode; a second electrode facing the first electrode; and an organic layer between the first electrode and the second electrode,
wherein the organic layer comprises an emission layer,
the emission layer comprises a first compound, a second compound, and a fluorescent dopant,
the first compound, the second compound, and the fluorescent dopant are different from one another,
a total weight of the first compound and the second compound is greater than a weight of the fluorescent dopant,
wherein
the first compound and the second compound satisfy at least one of Equation 1 to Equation 5, or the first compound, the second compound, and the fluorescent dopant satisfy at least one of Equation 6 to 11:
|S1(H1)−2×T1(H2)|≤0.15 eV Equation 1
T1(H1)>T1(H2) Equation 2
T1(H1)>T2(H2) Equation 3
|HOMO(H1)|<|HOMO(H2)| Equation 4
|LUMO(H1)|<|LUMO(H2)| Equation 5
S1(H2)>S1(FD) Equation 6
T1(H2)>T1(FD) Equation 7
|HOMO(H2)|>|HOMO(FD)| Equation 8
|LUMO(H2)|>|LUMO(FD)| Equation 9
|HOMO(H1)|>|HOMO(FD)| Equation 10
|LUMO(H1)|<|LUMO(FD)| Equation 11
wherein, in Equations 1 to 11,
S1(H1) represents an S1 singlet energy level (electron volts, eV) of the first compound,
S1(H2) represents an S1 singlet energy level (eV) of the second compound,
S1(FD) represents an S1 singlet energy level (eV) of the fluorescent dopant,
T1(H1) represents a T1 triplet energy level (eV) of the first compound,
T1(H2) represents a T1 triplet energy level (eV) of the second compound,
T1(FD) represents a T1 triplet energy level (eV) of the fluorescent dopant,
T2(H2) represents a T2 triplet energy level (eV) of the second compound, and
|HOMO(H1)| represents an absolute value of the highest occupied molecular orbital (HOMO) energy level (eV) of the first compound,
|HOMO(H2)| represents an absolute value of the HOMO energy level (eV) of the second compound,
|HOMO(FD)| represents an absolute value of the HOMO energy level (eV) of the fluorescent dopant,
|LUMO(H1)| represents an absolute value of the lowest unoccupied molecular orbital (LUMO) energy level (eV) of the first compound,
|LUMO(H2)| represents an absolute value of the LUMO energy level (eV) of the second compound,
|LUMO(FD)| represents an absolute value of the LUMO energy level (eV) of the fluorescent dopant, and
S1(H1), S1(H2), S1(FD), T1(H1), T1(H2), T1(FD), T2(H2), HOMO(H1), HOMO(H2), HOMO(FD), LUMO(H1), LUMO(H2) and LUMO(FD) are each be obtained using a density functional theory (DFT) that is a quantum chemistry computational method based on the 6-311+G(d,p) basis set, and
the first compound may be a compound represented by Formula 1:
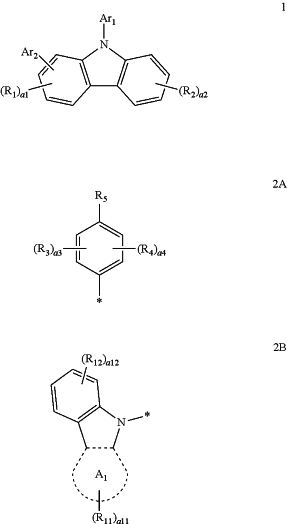 wherein, in Formula 1, Ar1 is a group represented by Formula 2A,
in Formula 1, Ar2 is a group represented by Formula 2B,
in Formula 2B, ring A1 is a dibenzofuran group or a dibenzothiophene group,
in Formulae 1, 2A, and 2B, R1 to R3, R11, and R12 are each independently:
hydrogen, deuterium, a C1-C20 alkyl group, a C1-C20 alkoxy group, a phenyl group, a biphenyl group, a terphenyl group, a carbazolyl group, a pyridinyl group, a dibenzofuranyl group, a dibenzothiophenyl group, or a dibenzosilolyl group;
a C1-C20 alkyl group or a C1-C20 alkoxy group, each substituted with at least one deuterium; or
a phenyl group, a biphenyl group, a terphenyl group, a carbazolyl group, a pyridinyl group, a dibenzofuranyl group, a dibenzothiophenyl group, or a dibenzosilolyl group, each substituted with at least one deuterium, a C1-C20 alkyl group, a C1-C20 alkoxy group, a phenyl group, a biphenyl group, a terphenyl group, a carbazolyl group, a pyridinyl group, a dibenzofuranyl group, a dibenzothiophenyl group, a dibenzosilolyl group, or any combination thereof,
in Formulae 1 and 2A, a1 and a3 are each independently an integer from 0 to 3, when a1 is 2 or greater, at least two R1(s) are identical to or different from each other, and when a3 is 2 or greater, at least two R3(s) are identical to or different from each other,
in Formulae 1 and 2B, a2 and a12 are each independently an integer from 0 to 4, when a2 is 2 or greater, at least two R2(s) are identical to or different from each other, and when a12 is 2 or greater, at least two R12(s) are identical to or different from each other,
in Formula 2B, a11 is an integer from 0 to 6, and when a11 is 2 or greater, at least two R11(s) are identical to or different from each other,
in Formula 2A, R4 is:
a phenyl group, a biphenyl group, or a terphenyl group;
a phenyl group, a biphenyl group, or a terphenyl group, each substituted with deuterium, a C1-C20 alkyl group, a C1-C20 alkoxy group, or any combination thereof,
in Formula 2A, a4 is an integer from 1 to 4, and when a4 is 2 or greater, at least two R4(s) are identical to or different from each other, and
in Formula 2A, R5 is:
hydrogen, deuterium, a C1-C20 alkyl group, or a C1-C20 alkoxy group; or
a C1-C20 alkyl group or a C1-C20 alkoxy group, each substituted with at least one deuterium,
wherein, in Formulae 2A and 2B, * indicates a binding site to an adjacent atom.
|