| CPC H01M 8/188 (2013.01) [C22C 1/00 (2013.01); C22C 27/06 (2013.01); H01M 4/38 (2013.01); H01M 8/04186 (2013.01); H01M 8/04276 (2013.01); H01M 8/04544 (2013.01); H01M 8/04611 (2013.01); H01M 8/04932 (2013.01); H01M 8/08 (2013.01); H01M 16/003 (2013.01); H01M 2004/8694 (2013.01); H01M 8/0693 (2013.01); H01M 2300/0002 (2013.01); H01M 2300/0005 (2013.01)] | 10 Claims |
|
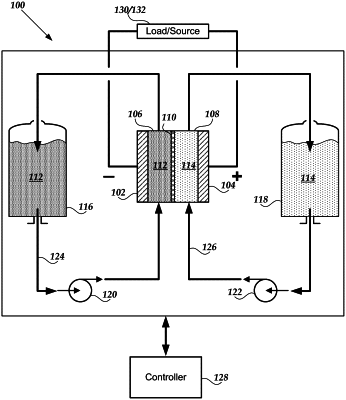
1. A method for preparation of electrolyte for a redox flow battery, the method comprising:
reducing chromium ore using a carbon source to convert the chromium ore to an iron/chromium alloy with carbon particles;
dissolving the iron/chromium alloy with carbon particles in sulfuric acid to form a first solution;
adding calcium chloride or barium chloride to the first solution to produce a second solution comprising FeCl3 and CrCl3; and
adding an acid to the second solution to form the electrolyte.
|