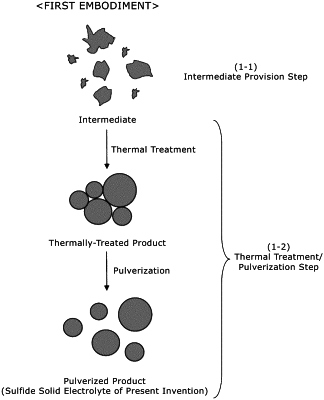
|
1. A sulfide solid electrolyte, comprising lithium (Li), phosphorus (P) and sulfur (S) elements, wherein the sulfide solid electrolyte has: a median diameter D50, which is a particle size at a cumulative volume of 50 vol % in a volume-based particle size distribution measured by a laser diffraction scattering particle size distribution measurement method, of 0.10 μm or more and 2.0 μm or less; a BET specific surface area of 2.0 m2/g or more and 20 m2/g or less; and a value of the formula: (A×B)/C, wherein A represents the BET specific surface area (m2/g), B re presents a true density (g/cm3), and C represents a CS value (m2/cm3), of 1.5 or more and 2.1 or less.
|