| CPC G16B 40/00 (2019.02) | 22 Claims |
|
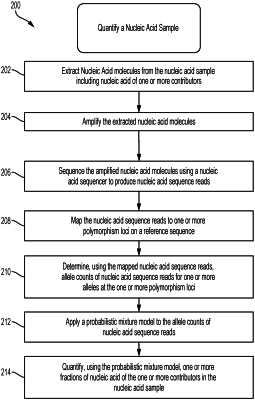
1. A method, implemented at a computer system that includes one or more processors and system memory, of quantifying a nucleic acid sample comprising tumor DNA and non-tumor DNA from a cell-free DNA (cfDNA) sample of an individual, the method comprising:
(a) mapping nucleic acid sequence reads generated from the nucleic acid sample to one or more alleles at one or more polymorphism loci on a reference sequence so as to identify reads among the nucleic acid sequence reads matching any sequence of a plurality of unbiased target sequences
(b) determining, using the nucleic acid sequence reads, allele counts for each of the one or more alleles at the one or more polymorphism loci;
(c) applying a probabilistic mixture model to the allele counts, wherein the probabilistic mixture model uses probability distributions to model the allele counts at the one or more polymorphism loci, the probability distributions comprising a first probability distribution accounting for errors in the nucleic acid sequence reads, a second probability distribution accounting for nucleic acid extraction errors, and a third probability distribution accounting for nucleic acid amplification errors and wherein the probabilistic mixture model quantifies fractions of nucleic acid of one or both of the tumor DNA or non-tumor DNA in the nucleic acid sample using a likelihood function comprising a product of likelihoods of non-stutter allele counts and likelihoods of stutter allele counts, and wherein the probabilistic mixture model prunes genotype configurations from data to be used to quantify the fractions of nucleic acid of the tumor DNA and non-tumor DNA in the nucleic acid sample, wherein pruning genotype configurations comprises limiting genotype configurations that are plausible by constructing a list of required alleles, wherein the list of required alleles consists essentially of alleles having allele counts above a threshold and too high to be plausible due to stutter drop-in, wherein the threshold is a sum of (i) a maximum non-stutter allele count, and (ii) a value multiplied by a count of potential stutter donor alleles;
(d) quantifying, using the probabilistic mixture model, a tumor DNA fraction in the nucleic acid sample; and
(e) determining a posterior probability that one or both of the tumor DNA fraction or a non-tumor DNA fraction has a specific genotype at the one or more polymorphism loci by performing steps comprising:
(i) multiplying prior probabilities of genotype configurations by likelihoods of the genotype configurations, wherein the prior probabilities are based on population data of allele frequencies of the genotype configurations;
(ii) normalizing a product of (i) by a sum over genotype space, wherein the genotype space is based on possible genotypes as a function of a relationship between the tumor DNA and non-tumor DNA; and
(iii) summing over genotype configurations containing the specific genotype to obtain the posterior probability; and
(f) developing and executing a plan for treatment of the individual based on the tumor DNA fraction in the nucleic acid sample from step (d) and the posterior probability of step (e), wherein executing the plan comprises administering a cancer treatment comprising one or more of surgically removing neoplastic cells from the individual, performing radiotherapy or causing radiotherapy to be performed on the individual, or administering or causing to be administered an anti-cancer drug to the individual.
|