| CPC C22B 23/043 (2013.01) [C01G 51/06 (2013.01); C01G 53/06 (2013.01); C22B 1/005 (2013.01); C22B 3/08 (2013.01); C22B 7/007 (2013.01); C22B 23/0453 (2013.01); C22B 26/12 (2013.01); H01M 10/54 (2013.01)] | 7 Claims |
|
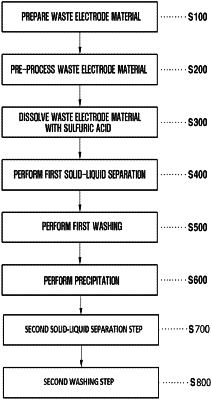
1. A method of recovering valuable metals by using a waste electrode material, the method comprising:
a waste electrode material preparation step of preparing a waste electrode material;
a pre-processing step of thermally processing the waste electrode material in a firing furnace in a temperature range of 300° C. to 500° C. for a duration of 30 minutes to 120 minutes;
a sulfuric acid dissolution step of, after the pre-processing step, adding sulfuric acid to the waste electrode material so that the waste electrode material reacts with the sulfuric acid;
a first solid-liquid separation step of separating the resulting product obtained through the sulfuric acid dissolution step into a metal melt and an unreacted residue;
a precipitation step of mixing lithium carbonate (Li2CO3) with the metal melt and stirring the resulting mixture; and
a second solid-liquid separation step of separating the resulting product obtained through the precipitation step into a precipitate and a filtrate,
wherein an aqueous solution of lithium sulfate is recovered from the filtrate, and nickel carbonate and cobalt carbonate are recovered from the precipitate.
|