| CPC C12N 5/0018 (2013.01) [A61K 9/06 (2013.01); A61K 9/1658 (2013.01); A61K 47/36 (2013.01); C12N 5/0625 (2013.01); C12N 5/0654 (2013.01); C12N 2501/105 (2013.01); C12N 2501/11 (2013.01); C12N 2501/115 (2013.01); C12N 2501/117 (2013.01); C12N 2501/12 (2013.01); C12N 2501/13 (2013.01); C12N 2501/135 (2013.01); C12N 2501/148 (2013.01); C12N 2501/15 (2013.01); C12N 2501/155 (2013.01); C12N 2501/165 (2013.01); C12N 2501/19 (2013.01); C12N 2501/22 (2013.01); C12N 2501/2301 (2013.01); C12N 2501/2306 (2013.01); C12N 2501/2308 (2013.01); C12N 2501/25 (2013.01); C12N 2502/1142 (2013.01); C12N 2502/1323 (2013.01)] | 27 Claims |
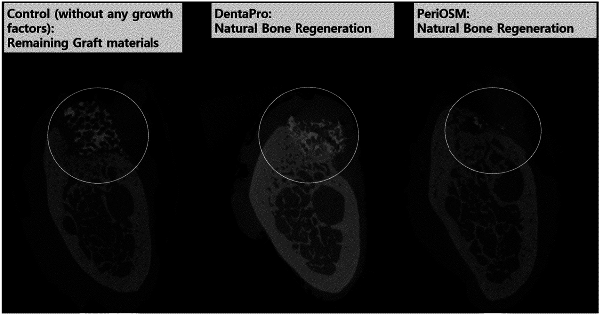
|
1. A method of treating a periodontal condition in a human subject, the method comprising administering to a periodontal site in need of a) gum and/or bone growth and/or b) gum erosion and/or bone resorption inhibition in the human subject an effective amount of a biocompatible composition comprising:
(a) a biocompatible polymer matrix, wherein the polymer matrix is agarose; and
(b) a conditioned cell medium comprising i) a cell culture medium and ii) one or more agents synthesized by and secreted from one or more cells cultured in the cell culture medium, wherein the cells are fibroblasts, dermal fibroblasts, neonatal dermal fibroblasts, osteoblasts, fibroblasts and osteoblasts cultured separately, and/or a co-culture of fibroblasts and osteoblasts.
|