| CPC C07D 491/18 (2013.01) [A61K 31/407 (2013.01); A61K 31/4353 (2013.01); A61K 31/436 (2013.01); A61K 47/62 (2017.08); A61K 47/64 (2017.08); A61P 35/00 (2018.01); C07D 498/18 (2013.01); C12N 9/90 (2013.01); C12P 17/18 (2013.01); C12Y 502/01008 (2013.01); A61K 38/00 (2013.01)] | 18 Claims |
|
1. A macrocyclic compound, or a pharmaceutically acceptable salt thereof, comprising: (a) a target protein interacting moiety; and (b) a presenter protein binding moiety; wherein the compound and a presenter protein form a complex that specifically binds to the target protein, wherein each of the compound and the presenter protein do not substantially bind to the target protein in the absence of forming the complex; or the compound and a presenter protein form a complex that binds to the target protein with at least 5-fold greater affinity than the affinity of each of the compound and the presenter protein to target protein in the absence of forming said complex;
wherein the target protein interacting moiety comprises the structure of Formula XIII:
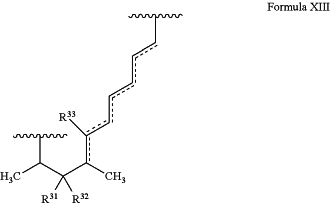 wherein the dotted lines represent zero to three double bonds, provided that no two double bonds are adjacent to one another;
R31 and R32 are independently hydrogen, hydroxyl, optionally substituted amino, halogen, thiol, optionally substituted amino acid, optionally substituted C1-C6 acyl, optionally substituted C1-C6 alkyl, optionally substituted C2-C6 alkenyl, optionally substituted C2-C6 alkynyl, optionally substituted C1-C6 heteroalkyl, optionally substituted C2-C6 heteroalkenyl, optionally substituted C2-C6 heteroalkynyl, optionally substituted C3-C10 cycloalkyl, optionally substituted C4-C10 cycloalkenyl, optionally substituted C4-C10 cycloalkynyl, optionally substituted C6-C10 aryl, optionally substituted C6-C10 aryl C1-C6 alkyl, optionally substituted C2-C9 heteroaryl, optionally substituted C2-C9 heteroaryl C1-C6 alkyl, optionally substituted C2-C9 heterocyclyl, optionally substituted C2-C9 heterocyclyl C1-C6 alkyl, or R31 and R32combine to form C═O; and
R33 is hydrogen or C═O, provided that no double bond is adjacent to a C═O; and
wherein the presenter protein binding moiety includes the structure of Formula I:
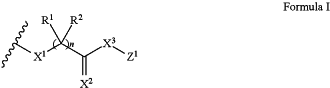 wherein n is 0 or 1;
X1 and X3 are each independently O, S, CR3R4, or NR5;
X2 is O, S, or NR5;
R1, R2, R3, and R4 are each independently hydrogen, hydroxyl, optionally substituted amino, halogen, thiol, optionally substituted C1-C6 alkyl, optionally substituted C2-C6 alkenyl, optionally substituted C2-C6 alkynyl, optionally substituted C1-C6 heteroalkyl, optionally substituted C2-C6 heteroalkenyl, optionally substituted C2-C6 heteroalkynyl, optionally substituted C3-C10 carbocyclyl, optionally substituted C6-C10 aryl, optionally substituted C6-C10 aryl C1-C6 alkyl, optionally substituted C2-C9 heteroaryl, optionally substituted C2-C9 heteroaryl C1-C6 alkyl, optionally substituted C2-C9 heterocyclyl, or optionally substituted C2-C9 heterocyclyl C1-C6 alkyl, or any two of R1, R2, R3, or R4 are taken together with the atom or atoms to which they are bound to form an optionally substituted carbocyclyl, optionally substituted heterocyclyl, optionally substituted aryl, or optionally substituted heteroaryl; and
each R5 is, independently, hydrogen, hydroxyl, optionally substituted C1-C6 alkyl, optionally substituted C2-C6 alkenyl, optionally substituted C2-C6 alkynyl, optionally substituted C1-C6 heteroalkyl, optionally substituted C2-C6 heteroalkenyl, optionally substituted C2-C6 heteroalkynyl, optionally substituted C3-C10 carbocyclyl, optionally substituted C6-C10 aryl, optionally substituted C6-C10 aryl C1-C6 alkyl, optionally substituted C2-C9 heteroaryl, optionally substituted C2-C9 heteroaryl C1-C6 alkyl, optionally substituted C2-C9 heterocyclyl, or optionally substituted C2-C9 heterocyclyl C1-C6 alkyl, or R5 and one of R1, R2, R3, or R4 are taken together with the atom or atoms to which they are bound to form an optionally substituted heterocyclyl or optionally substituted heteroaryl.
|