| CPC C07D 487/04 (2013.01) [B01J 21/04 (2013.01); B82Y 30/00 (2013.01)] | 7 Claims |
|
1. A process for preparing a compound of Formula (I):
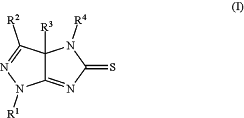 wherein:
R1 is H, halogen, alkyl, cycloalkyl, aryl, or heteroaryl;
R2 is H, halogen, alkyl, cycloalkyl, aryl, or heteroaryl;
R3 is H or C1-C3 alkyl; and
R4 is H, alkyl, cycloalkyl, aryl, or heteroaryl;
with the proviso that R2 is not pyrimidinyl or triazinyl;
wherein the process comprises the following steps:
(1) dissolving chitosan in acetic acid to obtain a chitosan solution;
(2) adjusting the pH of the chitosan solution formed in step (1) above to a pH in the range of 6 to 7;
(3) adding an aqueous suspension of aluminum oxide nanoparticles portion-wise to the chitosan solution formed in step (2) above, followed by stirring to form an aqueous mixture containing the chitosan solution and aluminum oxide nanoparticles;
(4) solution casting the aqueous mixture containing chitosan and aluminum oxide nanoparticles formed in step (3) above onto a carrier substrate, followed by drying to form a chitosan-alumina nanocomposite film, wherein the chitosan-alumina nanocomposite film is a heterogeneous base catalyst;
(5) reacting a compound of Formula (III):
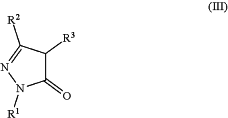 wherein:
R1 is H, halogen, alkyl, cycloalkyl, aryl, or heteroaryl;
R2 is H, halogen, alkyl, cycloalkyl, aryl, or heteroaryl; and
R3 is H or C1-C3 alkyl;
with the proviso that R2 is not pyrimidinyl or triazinyl;
with a compound of Formula (IV):
 wherein:
R4 is H, alkyl, cycloalkyl, aryl, or heteroaryl;
in the presence of ethanol and the chitosan-alumina nanocomposite film formed in step (4) above [10-20 wt % based on the weight of the compound of Formula (III)], to form a reaction mixture comprising ethanol and the heterogeneous base chitosan-alumina nanocomposite film catalyst;
(6) filtering the reaction mixture formed in step (5) above to remove the heterogeneous base chitosan-alumina nanocomposite film catalyst, followed by collecting a filtrate containing ethanol;
(7) evaporating ethanol from the filtrate collected in step (6) above, to precipitate the crude compound of Formula (I) above; and
(8) recrystallizing the crude compound of Formula (I) precipitated in step (7) above from ethanol, to form the compound of Formula (I):
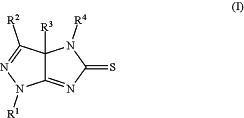 wherein:
R1 is H, halogen, alkyl, cycloalkyl, aryl, or heteroaryl;
R2 is H, halogen, alkyl, cycloalkyl, aryl, or heteroaryl;
R3 is H or C1-C3 alkyl; and
R4 is H, alkyl, cycloalkyl, aryl, or heteroaryl;
with the proviso that R2 is not pyrimidinyl or triazinyl.
|