| CPC C07D 405/04 (2013.01) [A61G 17/04 (2013.01); A61K 8/49 (2013.01); A61P 17/00 (2018.01); A61P 17/06 (2018.01); A61P 17/14 (2018.01); A61P 17/16 (2018.01); A61P 17/18 (2018.01); A61Q 19/02 (2013.01); C07D 221/16 (2013.01); C07D 491/048 (2013.01)] | 8 Claims |
|
1. A method of decreasing or reversing gain of skin pigmentation in a mammalian subject, the method comprising:
selecting a mammalian subject with pigmentary changes to the skin associated with a condition selected from the group consisting of oral contraceptive use, pregnancy, endogenous estrogens in females, solar lentigo, eczema, chemical burn scars, sun burn scars, thermal burn scars, lupus, psoriasis, sarcoidosis, pityriasis, erythema dyschromicum perstans, blistering diseases, drug reactions, and lichen planus;
topically or transdermally administering to the mammalian subject a pharmaceutical composition comprising at least one pharmaceutically acceptable excipient and a therapeutically effective amount of a PAQR7 (Progestin and AdipoQ Receptor 7) agonist, wherein the PAQR7 agonist is:
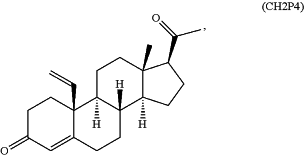 or a salt, solvate, tautomer, enantiomer, or diastereomer thereof; and
reducing the amount of melanin in the skin of the mammalian subject in a dose-dependent manner, wherein the PAQR7 agonist decreases or reverses gain of skin pigmentation in the mammalian subject, and wherein the CH2P4 does not bind to a canonical progesterone receptor (PR) in the mammalian subject.
|