| CPC C07D 403/04 (2013.01) [A61P 35/02 (2018.01); C07D 209/94 (2013.01); C07D 401/04 (2013.01); C07D 401/14 (2013.01); C07D 403/14 (2013.01); C07D 405/14 (2013.01); C07D 413/14 (2013.01); C07D 417/14 (2013.01); C07D 471/04 (2013.01); C07D 495/04 (2013.01)] | 30 Claims |
|
1. A compound of formula I:
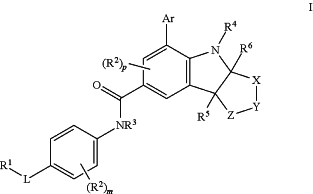 or an enantiomer or diastereomer, or a pharmaceutically acceptable salt thereof, wherein: R1 is —C1-6alkyl, cycloalkyl, or heterocycloalkyl;
each R2 is independently —H, -halo, —OH, —CN, —NH2, —NO2, —C1-6alkyl, or —C1-6alkoxy; wherein m is 0, 1, 2, 3, or 4; p is 0, 1 or 2;
R3 is —H or —C1-6alkyl;
R4 is —H, cycloalkyl, or —C1-6alkyl;
R5 and R6 are each independently —H or —C1-6alkyl;
Ar is aryl or heteroaryl;
X is —C(R7R7′)—;
Y is —C(R8R8′)—, —O—, —N(R10)—, —C(═O)—, —SO2;
Z is —[C(R9R9′)]n—; wherein n is 1, 2, 3, or 4;
or X and Y together form —(R7)C═C(R8)—;
L is —S—, —O—, —N(R11)—, —C(═O)N(R11)—, —C(═O)N(R11)—C1-4alkyl-, —N(R11)C(═O)—, —N(R11)C(═O)—C1-4alkyl-, —SO2N(R11)—, or —N(R11)SO2—;
each R7, R7′, R8, R8′, R9 and R9′ independently comprises:
1) —H,
2) —C1-6alkyl,
3) -cycloalkyl,
4) -heterocycloalkyl,
5) —OR12,
6) —N(R12)2,
7) —NR11C(═O)R12,
8) —NR11C(═O)OR12,
9) —NR11C(═O)N(R12)2,
10) —NR11SO2R12,
11) —NR11SO2N(R12)2,
12) —C(═O)R12,
13) —C(═O)O(C1-6 alkyl),
14) —C(═O)N(R12)2,
15) —SO2R12,
16) —SO2N(R12)2,
17) -aryl, and
18) -heteroaryl;
R10 is —H, —C1-6alkyl, -cycloalkyl, heterocycloalkyl, —C(═O)R12, —C(═O)OR12, —C(═O)N(R12)2, —SO2R12, —SO2N(R12)2, aryl, or heteroaryl;
each R11 is independently —H or —C1-6alkyl; and
each R12 is independently —H, —C1-6alkyl, —C1-6alkoxy, -cycloalkyl, heterocycloalkyl, aryl, or heteroaryl;
wherein at each occurrence alkyl is substituted with 0, 1, 2, 3, or 4 substituents comprising halo, -oxo, —OH, —CN, —NH2, —NO2, —SO2(C1-6alkyl), —C1-6alkoxy, -cycloalkyl, -heterocycloalkyl, aryl, and heteroaryl;
wherein at each occurrence cycloalkyl and heterocycloalkyl are substituted with 0, 1, 2, 3, or 4 substituents comprising halo, -oxo, —OH, —CN, —NH2, —NO2, —C1-6alkyl, —SO2(C1-6alkyl), —CO(C1-6alkyl), —C1-6 alkoxy, aryl, and heteroaryl;
wherein at each occurrence aryl and heteroaryl are substituted with 0, 1, 2, or 3 substituents comprising halo, —OH, —CN, —NH2, NO2, —NH(C1-6alkyl), —N(C1-6alkyl)2, —C1-6alkyl, -cycloalkyl, -heterocycloalkyl, and —C1-6 alkoxy.
|