| CPC C07D 319/12 (2013.01) [B01J 31/04 (2013.01); B01J 37/009 (2013.01); B01J 37/0236 (2013.01); B01J 37/031 (2013.01); B01J 37/04 (2013.01); B01J 37/06 (2013.01); B01J 2231/005 (2013.01); B01J 2531/002 (2013.01)] | 2 Claims |
|
1. A synthesis method of lactide by confinement effect catalysis of crystalline porous polymer material, comprising following steps:
step (I) synthesis of catalysts comprising:
a) placing a compound A, a compound B, mesitylene and 1,4-dioxane into a reaction vessel;
b) mixing the contents of the reaction vessel evenly;
c) adding acetic acid to the reaction vessel;
d) de-aerating the contents of the reaction vessel;
e) vacuum sealing the reaction vessel;
f) placing the reaction vessel in an oven for heating;
g) filtering precipitates from the reaction vessel;
h) washing the precipitates with a Soxhlet extractor;
i) vacuum drying the precipitates; and
j) obtaining solid catalysts;
wherein a structural formula of the compound A is:
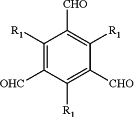 wherein R1 ═OH, CH3, OCH3, C2H5, F, Cl, Br or I;
wherein compound B is:
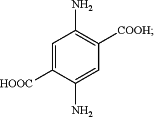 step (II) synthesis of lactide by confinement effect catalysis comprising:
a) adding the catalysts obtained in step (I), solvents, and lactic acid to a reaction vessel for reaction;
b) cooling down the reaction vessel slowly after the reaction;
c) filtering the contents of the reaction vessel after the reaction;
d) washing the contents of the reaction vessel after the reaction;
e) removing the solvents from the contents of the reaction vessel after the reaction at low pressure; and
f) obtaining crude lactide;
step (III) lactide purification comprising:
a) conducting liquid-liquid extraction on the crude lactide obtained in step (II) with methylbenzene and water,
b) combining organic phases of the liquid-liquid extraction,
c) removing the solvents of the combined organic phases at low pressure, and
d) obtaining lactide,
wherein in step (I) a mole ratio between the compound A and the compound B is 4:7; a volume ratio among the mesitylene, the 1,4-dioxane, and the acetic acid is 15:5:1; and a mole ratio between the compound A and the mesitylene is 1:25;
wherein in step (I) the method for de-aerating comprises freeze-pump-thaw cycling;
wherein in step (I) the conditions for heating in the oven comprise 80° C. for 3 days;
wherein in step (I) the conditions for washing with the Soxhlet extractor comprise specifically: washing four hours with THF and acetone; and conditions for vacuum drying comprise: 80° C. for 12 hours;
wherein lactic acid in step (II) comprises L-lactic acid of 90 percent purity;
wherein the solvents in step (II) comprise methylbenzene or ortho-xylene; wherein a mass ratio between the catalysts and the lactic acid is 1:10, and a weight/volume ratio of the catalysts and the solvents is 1:1 g/cm3;
wherein reaction conditions in step (II) comprise: reaction time of 5 hr and reaction temperature 120° C.;
wherein in step (II) washing is done by washing with acetonitrile.
|